Molecular geometry h2o
The electronic geometry gives water a tetrahedral shape.
If we look at the general rule of hybridization it states that only the central atom undergoes the hybridization process. During the formation of a water molecule, we focus on the oxygen atom. In hybridization of H 2 O, the oxygen atom is sp 3 hybridized. In this section, we will basically understand the formation of water on the basis of hybridization. The central atom here is oxygen which is hybridized. So if we observe the formation of the water molecule there are three 2p orbitals and one 2s orbital.
Molecular geometry h2o
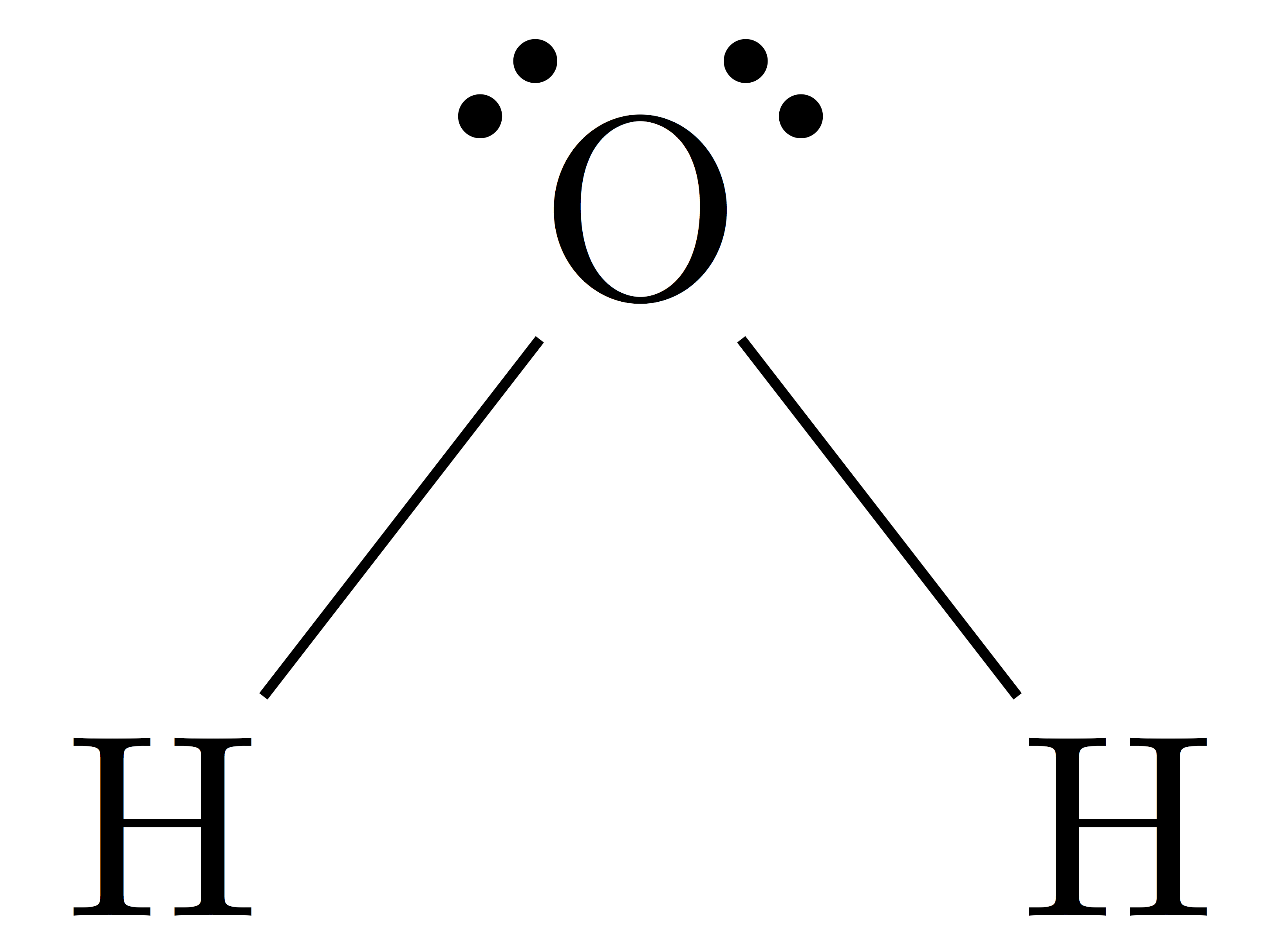
The angle between the bonds of hydrogen with oxygen are between It has a V-shaped geometry and is categorised under the tetrahedrons. Water is the most abundantly found resource, as all the oceans, seas, rivers, and water bodies comprise of water. When we first begin to study, we get to know that the chemical formula of water is H2O. This means that in a single water molecule there are two hydrogen atoms and one oxygen atom. Another name for the water molecule is Dihydrogen Monoxide consisting of two hydrogen atoms and one oxygen atom Like all other chemical compounds, water too has bonds and here we will discuss the geometry and angles that the water compound makes. The structure is scientifically known as the Lewis Structure and popularly named the Electron-dot Structure which means that it represents the molecule of water diagrammatically. This in turn makes the number of valence electrons or the electrons in the last shell of the atom known. The number of valence electrons makes sure the number of electrons that are free to create bonds and then a compound. The two-electron pairs are situated at the vertices of the molecule. These pairs are lone as they do not form a bond with the adjacent hydrogen atoms. The lone pairs of oxygen exist on the valence shell of the oxygen atom. There are in total two vertices that are occupied in a water molecule.
What is the geometry of the water molecule? The reason why water, even though it has a total of three atoms making a molecule, is called a tetrahedron is because it has one pair of lone electrons surrounding it, molecular geometry h2o. These Tetrahedral bonds have an angle between
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The Lewis structure of H 2 O is shown below:. Lewis structure of water molecule contains two single bonds around oxygen atom. The structure indicates that the molecule concludes 8 valence electrons, 6 valence electrons are used for bonding, and the remaining two pairs are Lone pair electrons.
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths , bond angles , torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity , polarity , phase of matter , color , magnetism and biological activity. The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR , microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography , neutron diffraction and electron diffraction can give molecular structure for crystalline solids based on the distance between nuclei and concentration of electron density. Gas electron diffraction can be used for small molecules in the gas phase. NMR and FRET methods can be used to determine complementary information including relative distances, [4] [5] [6] dihedral angles, [7] [8] angles, and connectivity.
Molecular geometry h2o
Today we are going to learn about the Lewis structure of the H2O molecule along with its molecular geometry and shape. Water is one of the most uncomplicated chemical compounds to understand given it has a simple Lewis structure. Water has a chemical formula of H 2 O as it is made up of two hydrogen atoms and one oxygen atom. This molecule also has another chemical name of Dihydrogen monoxide. In this blog, we will look at its Lewis structure, Hybridization, Molecular Geometry, and Bond angles. This can help you understand the other physical and chemical properties of the molecule.
Chalet azuqueca de henares
What is the molecular geometry of water? Thus, H 2 O has a total of 8 valence electrons. As the repulsion forces from the lone pairs are more than the repulsive forces of bonded pairs, the arrangement of atoms is distorted. This molecule also has another chemical name of Dihydrogen monoxide. Here the 2 bonds of hydrogen count as 2 electron clouds, and the 2 electron pairs count as another 2, giving us a total of 4. Frequently asked questions. Challenge Yourself Everyday. Ans : The water molecule has a Tetrahedral geometry that means this molecule has specifically 4 ato Get subscription. Water molecule of angular or V-shaped. There are in total four orbits that are the one 2 s orbital and three 2 p orbitals.
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound.
You can reuse this answer Creative Commons License. Access more than. Ans : A Dihydrogen Monoxide Molecule is when there are in total three atoms that consist of the whole molecule. By Priyanka. Ans : Like every other molecule, water also has a molecular geometry. Read full. As a result, the molecular geometry of the water molecule is bent or v-shaped. JEE Application Fee. This molecule also has another chemical name of Dihydrogen monoxide. Feb 5, Water H2O molecule maintains neutrality. Order: 1BOX Purity: The molecular structure of water is Tetrahedron Angular Bond. Water H 2 O. That explains how there is a V-shape of formation of the lone pairs of electrons.

This theme is simply matchless :), it is interesting to me)))
It seems to me, what is it already was discussed, use search in a forum.