Moles to moles calculator
The calculator below calculates the mass of the substance in grams or the quantity of the substance in moles, moles to moles calculator. Depending on the input data it can serve either as grams to moles calculator or as moles to grams calculator. It also displays the molar mass of the chemical substance and details of its calculation just for reference.
Want to know how to calculate moles? Need a grams-to-moles calculator or even a moles-to-grams calculator? Well, then, you've come to the right place. With our moles-to-grams converter, you can seamlessly convert between mass, molecular weight, and moles. Chemistry just became that little bit easier! Impress your friends with your astounding ability to find how many moles of a substance you have at a kilogram, ounce, or even tonne scale! Also useful for any serious industrial applications, for all you chemical engineers out there.
Moles to moles calculator
We've updated our Privacy Policy to make it clearer how we use your personal data. We use cookies to provide you with a better experience. You can read our Cookie Policy here. Molarity is the concentration of a solution in terms of the number of moles of the solute in 1 dm 3 1 liter of the solution. A mole is the quantity of anything that has the same number of particles as 12 g of carbon This equates to roughly 6. So, 1 mole of hydrogen gas H 2 contains 6. The molar mass is the mass in grams of 1 mole of a particular molecule. One mole of sodium Na is For sodium chloride NaCl they are in a ratio of so the molar mass of NaCl is For a compound like water H 2 O , 1 mole of hydrogen H is 1. Two solutions that have the same molarity will have the same number of molecules of the chemical per liter but are likely to contain differing masses of that chemical per liter to achieve this.
Molecular mass also called molecular weight - sum of the atomic weights of all atoms appearing in a given molecular formula.
Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units depending on the type of substance. As follows, we will extend the meaning of the coefficients in a chemical equation. The convention for writing balanced chemical equations is to use the lowest whole-number ratio for the coefficients. However, the equation is balanced as long as the coefficients are in a ratio.
Moles for chemists are not only found in gardens but appear everywhere: our mole calculator teaches you everything you need to know about this concept. A mole is a fundamental quantity in chemistry, slightly challenging our understanding but of utmost importance in every aspect of that science — and not only. And much more: keep reading to dive deep into the fundamental aspects of chemistry. Are you also interested in biology? Be sure to visit our protein molecular weight calculator , where you can learn a lot about the molecular weight of organic materials. We will introduce the concept of mole and gradually explain all the intricacies associated with it. A mole is a quantity of a substance that contains exactly an Avogadro's number of molecules or atoms.
Moles to moles calculator
Use the mole calculator below to find the quantity of a substance using a chemical formula or known molar mass. Joe is the creator of Inch Calculator and has over 20 years of experience in engineering and construction. He holds several degrees and certifications.
Amyrosebutt porn
Do you what to learn the importance of a molar ratio? The ratio of the coefficients is , which reduces to To determine the molar ratio from the molecular weight, you need the mass of each substance. Make an informed choice with our handy tool. Boiling Point Calculator. The convention for writing balanced chemical equations is to use the lowest whole-number ratio for the coefficients. Also useful for any serious industrial applications, for all you chemical engineers out there. The formula for converting molecules to moles is a little different than mass and volume because both molecules and moles are measures of quantity:. Calculating the molar ratio from a balanced reaction is the most straightforward method. Equipped with that knowledge, you can find the limiting agent in the reaction or which chemical is available in excess. Mass of the substance, grams. If you wanted to find the concentration of the hydrochloric acid, you could use our concentration calculator. Quantity of the substance, moles.
Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units depending on the type of substance. As follows, we will extend the meaning of the coefficients in a chemical equation.
While we're on the topic of chemistry, we have several other calculators that you might find useful. Join us in this article as we discuss the definition of a molar ratio, how to determine it using the molar ratio formula, and how to use it to get more information from your balanced chemical equation. Find out the molar mass of the substance hint: you can use Molar mass of the substance alone to calculate molar mass. First you must calculate the number of moles in this solution, by rearranging the equation No. For every two moles of sodium, one mole of chlorine is required to form two moles of sodium chloride, commonly known as table salt. From the coefficients in this balanced chemical reaction, we can derive the following information:. To determine the molar ratio between any two elements or compounds in a chemical reaction: Balance the chemical reaction. Review Checkmark. For the reaction in which hydrogen and oxygen combine to make water, for example, we can construct the following ratios:. List other known quantities.

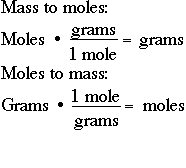
Such did not hear
In my opinion you commit an error. Let's discuss. Write to me in PM, we will communicate.