N2o lewis dot
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m.
Transcript: Let's do the N2O Lewis structure. N2O has 16 total valence electrons. There's three ways we can draw it, and all of them work pretty well. Let's take a look. For each structure, the atoms have fulfilled their octets, and for each Lewis structure, all the 16 valence electrons have been used. So we need to decide which one of these is going to be the best structure.
N2o lewis dot
I learned this by the counting-electron method, and then assigning formal charges to determine the most likely distribution of valence electrons. We have two nitrogens and one oxygen, which suggests that either we have oxygen in the middle or two nitrogens in a row. Notice how if you had oxygen in the middle , the formal charges of both nitrogens have no way of being distributed well without exceeding 8 electrons for oxygen :. Now we can get two plausible possibilities, which are both linear molecular geometries NOT bent!!! Two electron groups! Truong-Son N. Jan 26, Thus, one of the nitrogens must be in the middle. Related questions How is the Lewis structure of an ion written? Are non-valence electrons represented in a Lewis dot diagram? How is the total number of electrons represented in a Lewis structure determined? What are lone pairs and how are they represented in a Lewis dot diagram? Is it possible to draw Lewis dot diagrams for ionic compounds? How can I draw a Lewis dot diagram for carbon dioxide? Can Lewis structures predict the shape of a molecule?
Intro to Chemical Kinetics.
Post by » Mon Nov 05, am. Post by chaggard » Mon Nov 05, am. Post by » Tue Nov 06, am. Post by mbaker4E » Tue Nov 06, am. Post by Michael Nirula » Wed Nov 07, am. Laurence Lavelle Skip to content.
But have we ever tried to know more about this gas that can make humans laugh? I guess no! After gaining some knowledge about laughing gas, I decided to share it with you, so that next time we can laugh with knowledge!! N2O or nitrous oxide is commonly known as laughing gas. There are several other names by which this compound is known like sweet air, protoxide of nitrogen, etc. N2O is a colorless gas with a molecular weight of The boiling point of this compound is Nitrous oxide, from being used as an oxidizer in a rocket motor to its usage in internal combustion engines, has immense use in different fields. It is also used in aerosol propellants.
N2o lewis dot
N 2 O nitrous oxide has two nitrogen atoms and one oxygen atom. In the N 2 O Lewis structure, there is a triple bond between two nitrogen atoms, and a single bond between nitrogen and oxygen atom. The left nitrogen atom with a triple bond has one lone pair, and the oxygen atom with a single bond has three lone pairs. In the periodic table , nitrogen lies in group 15, and oxygen lies in group Hence, nitrogen has five valence electrons and oxygen has six valence electrons. Learn how to find: Nitrogen valence electrons and Oxygen valence electrons.
Chicas micro bikini
This is Dr. Having the appropriate skeletal structure and correct count of valence electrons, the goal is to place the electrons in bonds or lone pairs so that each atom has an octet of electrons. Example 1. Lewis Dot Structures: Exceptions. Especially with organic molecules, carbon atoms can constitute links in a chain, or backbone of central atoms. Power and Root Functions. Ksp: Common Ion Effect. The Colligative Properties. Post by mbaker4E » Tue Nov 06, am. Page updated The electrons involved in the N—O double bond, however, are in different positions: Two Lewis structures are shown.
This article discusses N2O lewis structure and its hybridization, shape, bond angle, and relevant detailed explanations.
Molecular Orbital Theory. Gamma Emission. Post by » Mon Nov 05, am. Writing Formulas of Coordination Compounds. Gas Evolution Equations. Introduction to Organic Chemistry. Hydrogen - as we have seen - is special since it can only accommodate a duet, and therefore can form at most one bond. Is it possible to draw Lewis dot diagrams for ionic compounds? Valence Electrons of Elements. The central and right structures are the same as the first, but the position of the double bonded oxygen has moved to the lower right oxygen in the central structure and to the top oxygen in the right structure. Chemistry Gas Laws. Subtract this number from the number of valence electrons for the neutral atom:. The Lewis symbols for the first 20 elements are shown in the adjacent figure. All oxygen atoms, however, are equivalent, and the double bond could form from any one of the three atoms. Crystal Field Theory: Octahedral Complexes.

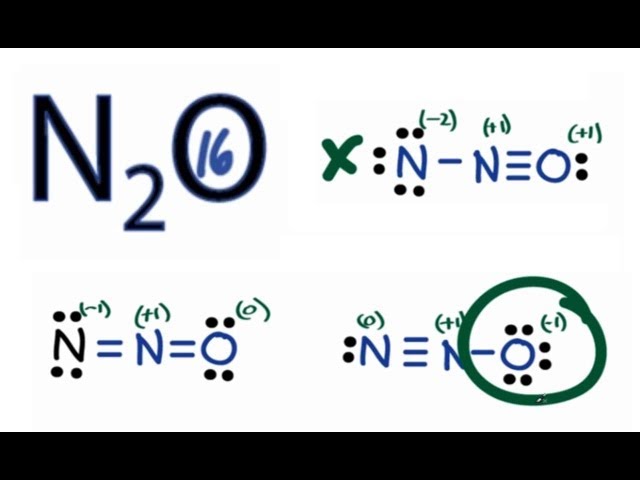
0 thoughts on “N2o lewis dot”