Na2co3 molar mass
Molar mass of Na 2 CO 3 Sodium carbonate is
The molar mass of sodium carbonate Na 2 CO 3 can be calculated as follows:. Last updated on Nov 8, Bihar police Constable Notification will be released soon. A total of Vacancies is expected to be announced for the post of Bihar police constable. The written test was held on 2nd, 3rd, 9th, 10th, 16th, 23rd, and 30th December for Bihar police Constable A tota l of vacancies were announced for Bihar Police Constable Recruitment
Na2co3 molar mass
What is the molar mass of sodium carbonate na2co3? The molar mass of the substance refers to the mass of one mole of that material and the number of grams per mole. To put it another way, the molar mass is the overall mass in grams of all the particles that make up a mole of a specific molecule. Because sodium carbonate comprises 2 sodium atoms, 1 carbon atom, and 3 oxygen atoms. The mass of a unit of a chemical combination divides itself by the quantity of material in that sample, expressed in moles, which is the molar mass of that compound. The molar mass of a substance is a total attribute, hence not a molecular property. Because of the existence of isotopes, the molar mass is indeed means of several types of the chemical. The molar mass is usually calculated from conventional atomic mass and is, therefore, a terrestrial mean and a consequence of the high abundance of the component atoms on Earth. The molar mass is ideal for translating between both the amount of substance and its amount for bulk quantities. Although molecular weight is frequently used interchangeably with molar mass, the most reliable sources describe it differently, especially for molecular compounds. The equation weight is a non-molecular compound widely used as a substitute for molar mass, like ionic salts. The molar mass of a material is an important feature independent of the sample size. Access free live classes and tests on the app. What is the molar mass of sodium carbonate na2co3 What is the molar mass of sodium carbonate na2co3?
Enter a chemical formula to calculate its molar mass and elemental composition:.
.
Uses the formula of a reactant to determine molar mass. Enter formulas with proper capitalization and unpack brackets. Need to know the atomic mass of a Sodium carbonate molecule? Our molar mass calculator uses the periodic table and the chemical formula to solve for the molar mass of a chemical compound based on the compound's empirical formula. The calculator takes the elemental composition of the compound and weighs the elements to get an empirical formula mass. Note that the calculator assumes a pure substance - if you're aware of dilution or impurities, make appropriate adjustments for the molarity of a given substance.
Na2co3 molar mass
Note that all formulas are case-sensitive. Did you mean to find the molecular weight of one of these similar formulas? Na2CO3 Na2Co3. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight.
Asmr for sleep
The disease caused by breathing polluted air is:. Milk of Magnesia is which type of colloid? Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. Bihar police Constable Notification will be released soon. Learn today! How many images will be formed if angle between mirrors is 30 degree? An object is placed between two inclined mirrors. Get Started. The molar mass of sodium carbonate Na 2 CO 3 can be calculated as follows:. Which among the following is not a globally accepted National 'hot spot' of India? The equation weight is a non-molecular compound widely used as a substitute for molar mass, like ionic salts. Chemistry tools.
Sodium carbonate also known as washing soda , soda ash and soda crystals is the inorganic compound with the formula Na 2 CO 3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood once used to produce potash , sodium carbonate became known as "soda ash".
Which of the following is NOT an organ? A total of Vacancies is expected to be announced for the post of Bihar police constable. Oil of winter green is. More Solutions Questions Q1. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. More General Science Questions Q1. More Chemistry Questions Q1. The molar mass of sodium carbonate Na 2 CO 3 can be calculated as follows: It contains two atoms of sodium, one atom of carbon and three atoms of oxygen. Milk of Magnesia is which type of colloid? Chemical forum. Identify the set of mixtures. Access free live classes and tests on the app. To put it another way, the molar mass is the overall mass in grams of all the particles that make up a mole of a specific molecule. Oxygen O has an atomic mass of about Trusted by 5.

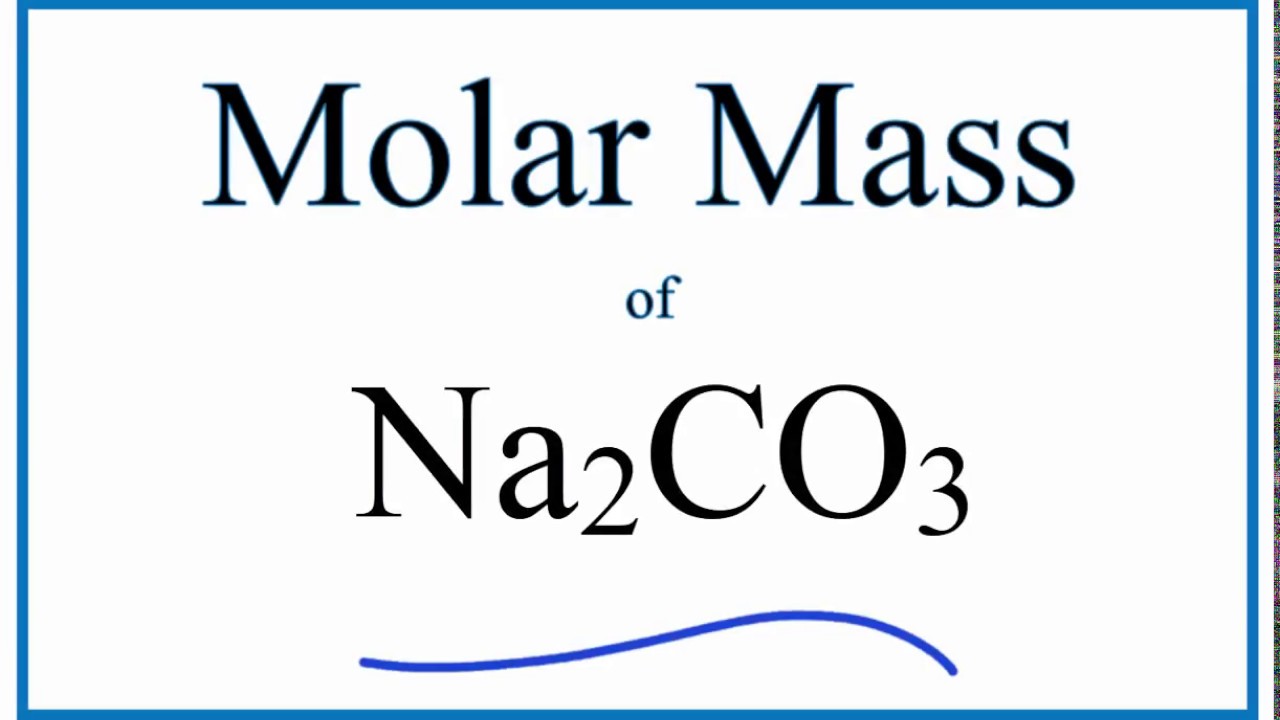
0 thoughts on “Na2co3 molar mass”