Nacl boiling point
Solution A contains 1 mole of sodium chloride in 1 lite of wats? Solution B contains 1 mole of potassium chlorice in 1 lite of water. Both solutions are heated and the boiling point is measured.
See more Sodium products. Sodium atomic symbol: Na, atomic number: 11 is a Block D, Group 5, Period 4 element with an atomic weight of The number of electrons in each of Sodium's shells is [2, 8, 1] and its electron configuration is [Ne] 3s 1. The sodium atom has a radius of Sodium was discovered and first isolated by Sir Humphrey Davy in In its elemental form, sodium has a silvery-white metallic appearance.
Nacl boiling point
If you add salt to water, you raise the water's boiling point, or the temperature at which it will boil. The temperature needed to boil will increase about 0. This is an example of boiling point elevation , and it is not exclusive to water. It occurs any time you add a nonvolatile solute such as salt to a solvent such as water. Water boils when the molecules are able to overcome the vapor pressure of the surrounding air to move from the liquid phase to the gas phase. When you add a solute that increases the amount of energy heat needed for water to make the transition, a few processes occur. When you add salt to water, sodium chloride dissociates into sodium and chlorine ions. These charged particles alter the intermolecular forces between water molecules. In addition to affecting the hydrogen bonding between water molecules, there is an ion-dipole interaction to consider: Every water molecule is a dipole, which means one side the oxygen side is more negative and the other side the hydrogen side is more positive. The positively charged sodium ions align with the oxygen side of a water molecule, while the negatively charged chlorine ions align with the hydrogen side. The ion-dipole interaction is stronger than the hydrogen bonding between the water molecules, so more energy is needed to move water away from the ions and into the vapor phase. Even without a charged solute, adding particles to water raises the boiling point because part of the pressure the solution exerts on the atmosphere now comes from solute particles, not just solvent water molecules.
Scientific Notation. Molecular Geometry. Calculating K For Overall Reaction.
Product Number: All applicable American Elements product codes, e. Supplier details: American Elements Weyburn Ave. None -. SKIN: Wash off thoroughly with soap and water. In severe cases seek medical attention. Induce vomiting if conscious. Obtain medical attention.
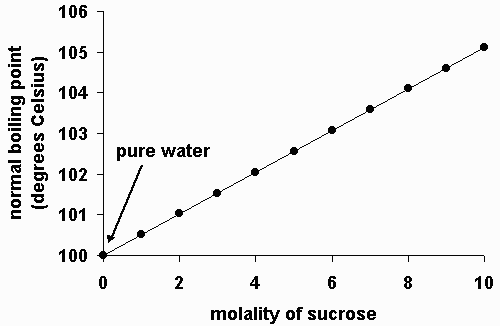
Many of the physical properties of solutions differ significantly from those of the pure substances discussed in earlier chapters, and these differences have important consequences. Aqueous solutions have both a lower freezing point and a higher boiling point than pure water. This solute lowers the freezing point of the water, preventing the engine from cracking in very cold weather from the expansion of pure water on freezing. Changes in the freezing point and boiling point of a solution depend primarily on the number of solute particles present rather than the kind of particles. As we will see, the vapor pressure and osmotic pressure of solutions are also colligative properties. When we determine the number of particles in a solution, it is important to remember that not all solutions with the same molarity contain the same concentration of solute particles. Consider, for example, 0. Because sucrose dissolves to give a solution of neutral molecules, the concentration of solute particles in a 0.
Nacl boiling point
Sodium chloride. No predicted properties have been calculated for this compound. We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. NaCl [Formula]. Caswell No. D [DBID]. Incompatible with strong oxidizing agents.
Where can i buy soursop leaves near me
Precipitation: Ksp vs Q. Gamma Emission. Naming Ethers. Diprotic Acids and Bases Calculations. Calculating K For Overall Reaction. Aldehydes and Ketones Reactions. Density of Non-Geometric Objects. Le Chatelier's Principle. Protective gloves made of polyvinyl alcohol PVA are required. Solubility Rules. Sodium atomic symbol: Na, atomic number: 11 is a Block D, Group 5, Period 4 element with an atomic weight of ChuHtet August 18, , pm 1. Periodic Trend: Metallic Character.
This page explains the relationship between the arrangement of the ions in a typical ionic solid like sodium chloride and its physical properties - melting point, boiling point, brittleness, solubility and electrical behavior.
Boron Family: Borane. Quantum Numbers: Number of Electrons. Significant Figures: In Calculations. Sodium Hexachloroplatinate IV Hexahydrate. Naming Alkenes. Materials by Application. Additional technical, research and safety MSDS information is available as is a Reference Calculator for converting relevant units of measurement. Mole Fraction. Periodic Table: Phases. Contact your local waste disposal authority for advice, or pass to a chemical disposal company. The sodium atom has a radius of Molecular Polarity. Cell Potential: The Nernst Equation. Skip to main content.

0 thoughts on “Nacl boiling point”