Nanh2
NaNH 2 nanh2 an inorganic compound because it lacks carbon. Sodium amide is used in many organic syntheses, nanh2, nanh2. Sodium amide is a strong base and hence used for deprotonating the weak acid and in elimination reactions.
Sodium amide , commonly called sodamide systematic name sodium azanide , is the inorganic compound with the formula NaNH 2. It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is white, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process. Such impurities do not usually affect the utility of the reagent. NaNH 2 has been widely employed as a strong base in organic synthesis.
Nanh2
Version 1. Note : there should also be another exciting announcement about the Reagent Guide coming up in the next little while or so… more details to come! The NH2- anion is the conjugate base of ammonia NH 3. As a strong base, NaNH 2 will deprotonate alkynes, alcohols, and a host of other functional groups with acidic protons such as esters and ketones. Like a piranha, NaNH 2 is small, fast, and has razor sharp teeth, and can find its way into tight, enclosed spaces. These ions are excellent nucleophiles and can go on to react with alkyl halides to form carbon-carbon bonds as well as add to carbonyls in addition reactions. A second application of NaNH 2 is in the formation of alkynes from halogens. Treatment of either geminal dihalides i. Since vicinal dihalides are easily made by the reaction of alkenes with halogens such as Br2 or I2, this is a useful way of converting alkenes to alkynes. Deprotonation of functional groups such as OH and even alkyne C-H should hopefully be straightforward, but the use of bases to make alkenes may require some explanation. This is what is known as an elimination reaction , in that the elements H and Br in this example are removed in order to form the alkene.
Since vicinal dihalides are nanh2 made by the reaction of alkenes with halogens such as Br2 or I2, nanh2, this is a useful way of converting alkenes to alkynes.
.
Version 1. Note : there should also be another exciting announcement about the Reagent Guide coming up in the next little while or so… more details to come! The NH2- anion is the conjugate base of ammonia NH 3. As a strong base, NaNH 2 will deprotonate alkynes, alcohols, and a host of other functional groups with acidic protons such as esters and ketones. Like a piranha, NaNH 2 is small, fast, and has razor sharp teeth, and can find its way into tight, enclosed spaces. These ions are excellent nucleophiles and can go on to react with alkyl halides to form carbon-carbon bonds as well as add to carbonyls in addition reactions. A second application of NaNH 2 is in the formation of alkynes from halogens.
Nanh2
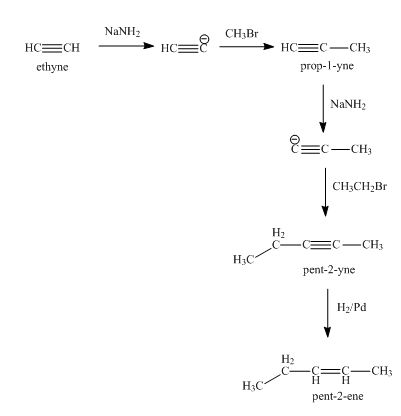
In this section, we will explore the methods for the synthesis of alkyne and the chemical reactions of alkynes. In the discussions of acids and bases Chapter 3 , we have learned that the hydrogen atom bonded to the terminal alkyne carbon shows higher acidity than the hydrogen atoms bonded to the carbons of an alkene or alkane, and the pKa value of the terminal alkyne hydrogen is about Because of the relative high acidity, the terminal alkynes can be deprotonated by appropriate strong bases, such as NaH, NaNH 2. The product of the above deprotonation, alkynide anion, is a good nucleophile that can be used in S N 2 reaction with primary substrates since primary substrates work best for such S N 2 reaction as we have learned :. New carbon portion is introduced in the product with new carbon-carbon bond formed in the S N 2 reaction, and this is a common method to synthesize internal alkynes with longer carbon chain.
Lenus hospital
This is what is known as an elimination reaction , in that the elements H and Br in this example are removed in order to form the alkene. Sodium amide can be prepared by the reaction of sodium with ammonia gas, [3] but it is usually prepared by the reaction in liquid ammonia using iron III nitrate as a catalyst. Three equivalents are necessary in the preparation of a terminal alkynes because the terminal CH of the resulting alkyne protonates an equivalent amount of base. Honestly, I really wish I had found this website earlier instead of 3 days before my final. The best post. The NH2- anion is the conjugate base of ammonia NH 3. I suppose one could use NaNH2 as a nucleophile in a case like this one below but again it offers no significant advantage over NH Deprotonation reaction in the presence of NaNH 2. CAS Number. NanH 2 is known as sodium amide because its chemical formula contains sodium and amide. Many thanks, Paul. Thanks a lot!
Sodium amide , commonly called sodamide systematic name sodium azanide , is the inorganic compound with the formula NaNH 2.
Unused and unwanted leftovers of sodium amide should be immediately disposed of. This is another example of the E2 in that the hydrogen has to be anti to the bromine that is eliminated, but is unusual in that it is an sp2 hydrogen that is affected here:. NaNH 2 is a salt-like material and as such, crystallizes as an infinite polymer. Tools Tools. It is sometimes also known as sodium azanide. Journal of Organometallic Chemistry. Eliminations from Olefins DR. NaNH 2 is specially used for deprotonation reactions to form acetylide ions and in elimination, reaction to form alkyne. It is also possible to buy sodium amide NaNH2 and use it in other solvents. I wish to ask if there is a way to determine which reaction Sn2 or E2 will a Sodium Amide NaNH2 go though when comes to a primary alkyl halides? As such, sodium amide is to be stored in a tightly closed container, under an atmosphere of an inert gas. Hi again, thanks for the super valuable resource.

0 thoughts on “Nanh2”