Nh43po4
Ammonium phosphate is an unstable compound made of ammonium and phosphate salt with the chemical formula NH 4 3 PO 4. Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. Considerable nh43po4 is generated which transform the ammonium phosphate to the molten state. It includes a group of nitrogen phosphorus nh43po4 mono ammonium phosphates and diammonium phosphates, nh43po4, mixtures of the two or combinations with ammonium nitrate or ammonium sulfate, nh43po4.
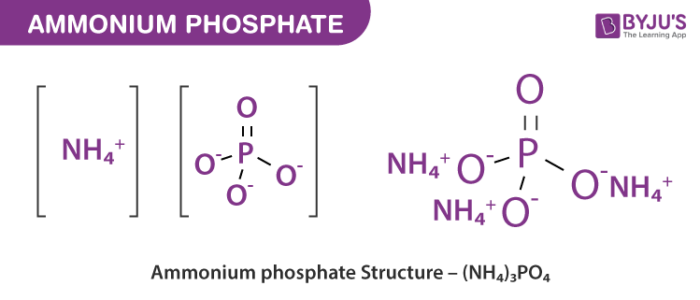
Ammonium phosphate is the inorganic compound with the formula NH 4 3 PO 4. It is the ammonium salt of orthophosphoric acid. A related "double salt", NH 4 3 PO 4. NH 4 2 HPO 4 is also recognized but is impractical to use. Both triammonium salts evolve ammonia. In contrast to the unstable nature of the triammonium salts, the diammonium phosphate NH 4 2 HPO 4 and monoammonium salt NH 4 H 2 PO 4 are stable materials that are commonly used as fertilizers to provide plants with fixed nitrogen and phosphorus.
Nh43po4
Ammonium phosphate is an unstable compound made of ammonium and phosphate salt with the chemical formula NH4 3PO4. Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. Considerable heat is generated which transform the ammonium phosphate to the molten state. It includes a group of nitrogen phosphorus materials: mono ammonium phosphates and diammonium phosphates, mixtures of the two or combinations with ammonium nitrate or ammonium sulfate. Ammonium phosphate is used as a leavening agent during the process of bakingIts interaction with heat, results in instant evaporation without leaving any residue of ammonia. Due to the ability of rapidly dissolving and becoming soluble, ammonium phosphate works as an effective fertilizer. It has two crucial elements embedded in them, the ammonium and phosphate, both of which are highly beneficial to the plants. It is used as an intermediate for the synthesis of pesticides such as triazolone, triadimenol and vinyl azoles Nickel carbonate finds applications in sulphamate baths, metal phosphating, electroplating, and ceramic processes. This article will focus on its utilization as a catalyst
Xylene C 8 H Acetic Acid Chemical Formula.
Then, lookup atomic weights for each element in periodic table : P: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite?
Ammonium phosphate is an unstable compound made of ammonium and phosphate salt with the chemical formula NH 4 3 PO 4. Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. Considerable heat is generated which transform the ammonium phosphate to the molten state. It includes a group of nitrogen phosphorus materials: mono ammonium phosphates and diammonium phosphates, mixtures of the two or combinations with ammonium nitrate or ammonium sulfate. Other names — triammonium phosphate, diazanium hydrogen phosphate. Ammonium phosphate is a high source of elemental nitrogen used as an ingredient in certain fertilizers.
Nh43po4
Ammonium phosphate is a salt that is made up of ammonia and phosphorus, and its chemical formula is NH 4 3 PO 4. However, this is a very unstable salt and due to how unstable it is, it is not exactly a salt worth a lot of commercial value. It can be formed by combining phosphoric acid along with ammonia, or by adding a lot more ammonia with acid phosphate. For it to be used commercially, it is mostly obtained from crystalline powders. The molecular formula for this salt is NH 4 3 PO 4 and it is also referred to as triammonium phosphate or diazonium hydrogen phosphate. When phosphoric acid H 3 PO 4 reacts with ammonia NH 3 it needs to be anhydrous in nature , we get the following reaction:. On combining concentrated quantities of both the products, ammonium phosphate is amassed in the form of a crystallised powder. This powder is soluble in water and if the water is boiled, then the solution loses the ammonia in the form of gas. Image Will be Uploaded Soon. Every salt or compound has a set of distinct physical and chemical properties which are used as basic determinants to differentiate it from other chemical compounds and salts.
Usmc new tattoo policy
GdPO 4. FREE Signup. Nitrogen Dioxide NO 2. TmPO 4. Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. Examples of molecular weight computations: C[14]O[16]2 , S[34]O[16]2. Download as PDF Printable version. You can help Wikipedia by expanding it. Mar 13, Is argon flammable? Put your understanding of this concept to test by answering a few MCQs. In the other side, it is ionized to ammonium.
Molecular weight calculation: Note that all formulas are case-sensitive.
Ammonium phosphate can also be used as a great ingredient for dry chemical extinguisher. ISBN Ammonium phosphate is an unstable compound made of ammonium and phosphate salt with the chemical formula NH 4 3 PO 4. Signal word. View Result. Ammonium phosphate is a high source of elemental nitrogen used as an ingredient in certain fertilizers. How to cite? Chemical compound. Download as PDF Printable version. Find atomic masses: look up the atomic masses of each element present in the compound. Resorcinol C 6 H 6 O 2. This article will focus on its utilization as a catalyst

0 thoughts on “Nh43po4”