Number of structural isomers possible in c3h6o
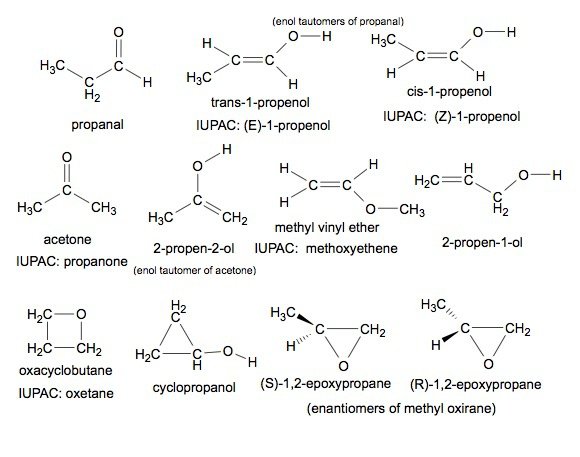
Sign in Open App. Most Upvoted Answer. Prop1en1ol, 2. Prop1en2ol, 3.
Assertion : Carbon oxygen bond length of phenol is slightly less than that in methanol. Reason : There exist a partial double bond character and carbon to which oxygen is attached in phenol is s p 2 hybridised. An important route to unsymmetrical ethers is a nucleophilic substitution reaction known as the Williamson synthesis. This synthesis consists of an S N 2 reaction of a sodium alkoxide with an alkyl halide, alkyl sulphonate or alkyl sulphate. By a proper choice of reagents, both symmetrical and unsymmetrical ethers can be prepared by Williamson synthesis. The reverse process of cleavage of ethers to give back the original alkyl halide and the alcohol can be carried out by heating the ether with HI at K. Which of the following reagents when heated will give a good yield of ether?
Number of structural isomers possible in c3h6o
.
The final product X in the following reaction sequence is?
.
A series of compounds in which successive members differ from one another by a CH 2 unit is called a homologous series. It is important that you commit to memory the names of the first 10 straight-chain alkanes i. You will use these names repeatedly when you begin to learn how to derive the systematic names of a large variety of organic compounds. You need not remember the number of isomers possible for alkanes containing more than seven carbon atoms. Such information is available in reference books when it is needed. When drawing isomers, be careful not to deceive yourself into thinking that you can draw more isomers than you are supposed to be able to. Remember that it is possible to draw each isomer in several different ways and you may inadvertently count the same isomer more than once. Alkanes are organic compounds that consist entirely of single-bonded carbon and hydrogen atoms and lack any other functional groups. Alkanes are often called saturated hydrocarbons because they have the maximum possible number of hydrogens per carbon.
Number of structural isomers possible in c3h6o
After completing this section, you should be able to explain the differences among constitutional structural isomers and stereoisomers geometric isomers. The following flow chart can be used to identify the relationship of two compounds with respect to isomerization:. Each sp 3 hybrid orbital is cylindrically symmetrical all cross-sections are circles , resulting in a carbon—carbon single bond that is also cylindrically symmetrical about the C—C axis.
Ducati panigale front view
Methoxyethene, 5. Sign in Open App. How many structural isomers are possible in C3H7Cl? Cyclic ketone: A cyclic ketone is an isomer where the carbon atoms form a ring, and the carbonyl group is attached to one of the carbon atoms in the ring. How many structural isomers are possible for C3H6O Cumene on reaction with oxygen followed by treatment with H 2 SO 4 g There are two possible positions for the carbonyl group: in the middle of the main chain or in the middle of the branch. Assertion : Carbon oxygen bond length of phenol is slightly less than that in methanol. If it had been C3H6O therefore the similar no of isomer will be formed.. Answer this doubt.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Donate Log in Sign up Search for courses, skills, and videos.
Scan this QR code to download the app for Free. Community Answer. Propanone, 7. Create you account for free. Free Exam Preparation at your Fingertips! Select a course to view your unattempted tests. Methoxyethene, 5. H2O greyish green? Signup with Email. Create Account. View answers on App. In this case, the carbon chain contains three carbon atoms with the aldehyde group attached to one end. Scan the QR code to for best learning experience! There are two possible positions for the carbonyl group: in the middle of the main chain or in the middle of the branch. Find important definitions, questions, meanings, examples, exercises and tests below for No.

Quite right! So.
Your phrase is magnificent
It agree, the useful message