Number of valence electrons in phosphorus
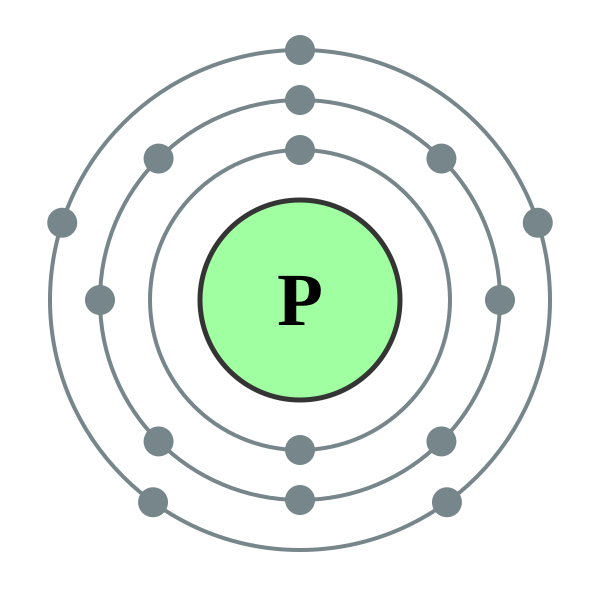
Valence electrons in a Phosphorus atom Therefore, the valence electron in a Phosphorus atom is 5. Byju's Answer.
There are two ways to find out. Either you take a look at your periodic table and look at which group number P belongs this can be seen on upper portion or you can draw the electron configuration of P atom based on its atomic number which is According to the periodic table above, phosphorus belongs to Group 5A. Therefore, Its valence electrons should be 5. Thus, valence electrons for P is 5.
Number of valence electrons in phosphorus
Contining on from CHM there are several topics that you must have a firm grasp on in order to be able to understand the concepts being presented in CHM An atom is made up of protons, neutrons and electrons. Protons and Neutrons are located in the nucleus of the atom and electrons are located in shells surrounding the nucleus. An elements atomic number is equal to the number of protons located in its nucleus. If you change the number of protons, you change the element you are talking about. The atomic mass of an element is equal to the mass of its protons plus its neutrons. From the mass in the periodic table and the atomic number, you should be able to determine the number of neutrons in the atom. The number of electrons in an atom is always equal to the number of protons so long as the atom is neutral. Negatively charged atoms are called anions and positively charged atoms are called cations. Ions form to increase the stability of the atom. Group VIII elements, the noble gases, are the most stable elements and have eight valence electrons outermost shell electrons. All of the other elements in Groups I -VII form ions and bonds in an effort to obtain eight electrons in their outermost shell. Example: Nitrogen is a Group V element. In order to become like the noble gas Neon, it must gain 3 electrons. Thus when Nitrogen forms ions, they have a 3- charge and when it forms bonds it generally bonds to three other elements.
Contining on from CHM there are several topics that you must have a firm grasp on in order to be able to understand the concepts being presented in CHM The next largest atom, beryllium, has 4 electrons, so its electron configuration is 1 s 2 2 s 2, number of valence electrons in phosphorus. However, a curious thing happens after the 3 p subshell is filled: the 4 s subshell begins to fill before the 3 d subshell does.
Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons occupy the space about the nucleus. Do they move around the nucleus at random, or do they exist in some ordered arrangement? The modern theory of electron behavior is called quantum mechanics. It makes the following statements about electrons in atoms:. It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that.
Phosphorus is the fifteenth element in the periodic table. Phosphorus is the element in group Phosphorus is an essential chemical element that has a wide variety of uses. It is one of the most important elements in the body, and it plays a vital role in many biological processes. Phosphorus is found in every cell of the human body, and it plays a key role in metabolism, energy production, and cell growth. It also helps to maintain healthy bones and teeth. Phosphorus is also used for industrial purposes. It can be used as an additive to fertilizers to increase crop yields, or as an ingredient in detergents to make them more effective.
Number of valence electrons in phosphorus
After completing this section, you should be able to write the ground-state electron configuration for each of the elements up to and including atomic number The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals.
Rv rentals in south dakota
Valence electrons are in the highest-numbered shell; all other electrons are core electrons. There are two ways to find out. That gives a total of 5 electrons, so neutral phosphorus atoms have 5 valence electrons. Negatively charged atoms are called anions and positively charged atoms are called cations. Key Takeaway Electrons are organized into shells and subshells about the nucleus of an atom. Other molecules, covalent molecules, do not dissociate into parts in water and are therefore considered non-polar. An elements atomic number is equal to the number of protons located in its nucleus. A neutral phosphorus atom has 15 electrons. Solution A neutral phosphorus atom has 15 electrons. There are some basic rules for drawing Lewis structures that you should be familiar with:.
There are two ways to find out. Either you take a look at your periodic table and look at which group number P belongs this can be seen on upper portion or you can draw the electron configuration of P atom based on its atomic number which is
Sign in. The central atom in a molecule is usually the least electronegative atom. Nikka C. Valence electrons in a Phosphorus atom What is the difference between core electrons and valence electrons? Valence electrons are in the highest-numbered shell; all other electrons are core electrons. Key Takeaway Electrons are organized into shells and subshells about the nucleus of an atom. Protons and Neutrons are located in the nucleus of the atom and electrons are located in shells surrounding the nucleus. The 10 remaining electrons, from the first and second shells, are core electrons. An atom of phosphorus has the symbol 31 15 P. Thus, it is convenient to separate electrons into two groups. Lewis structures are constructed in order to satisfy the octet rule for each of the atoms in a molecule.

You are not right. I am assured. I suggest it to discuss. Write to me in PM, we will communicate.
I apologise, but, in my opinion, you are mistaken. I suggest it to discuss.