Oxidation number of n in hno3
In comparing the chemistry of the amines with alcohols and ethers, we discover many classes of related compounds in which nitrogen assumes higher oxidation states, in contrast to limited oxidation states of oxygen.
If we multiply these by how many of that atom we have in the compound, the number left is. Oxygen is typically stackrel -II O , and it is so here And we remember our definition of oxidation numbers. Oxidation number is the charge left on the atom of interest when all the bonding pairs of electrons are broken, with the charge going to the MOST electronegative atom. Chemistry Electrochemistry Oxidation Numbers. Nathan L. May 24,
Oxidation number of n in hno3
Wiki User. There are two oxidation numbers. HNO3 already has hydrogen and nitrogen in their highest possible oxidation states. So sum is zero. It shows some oxidation numbers. No, the can only have positive oxidation numbers. All metals have positive oxidation numbers. Non-metals may have positive or negative oxidation numbers and some metalloids have both positive and negative oxidation numbers as well. Tags Oxidation Numbers Subjects. Log in. Study now See answer 1.
This has led to a spin labeling strategy for investigating the conformational structures of macromolecules like proteins. Related questions.
What is the oxidation number of N in H N O 4? Nitrous oxide N 2 is. What is the oxidation state of nitrogen in H N O 3. What is the oxidation number of nitrogen in a nitric acid b nitrous acid c nitric oxide d nitrous oxide e ammonia f N 2? What is the oxidation state of N in H N O 3? The oxidation number of N nitric acid molecule is.
If we multiply these by how many of that atom we have in the compound, the number left is. Oxygen is typically stackrel -II O , and it is so here And we remember our definition of oxidation numbers. Oxidation number is the charge left on the atom of interest when all the bonding pairs of electrons are broken, with the charge going to the MOST electronegative atom. Chemistry Electrochemistry Oxidation Numbers.
Oxidation number of n in hno3
What is the oxidation number of N in NO 3 -? Byju's Answer. Open in App. The oxidation state or oxidation number can be defined as the loss or gain of electrons from an atom, molecule, or ion to achieve a stable electronic configuration. Each element shows a different oxidation state depending upon the total number of valence shell electrons. Nitrogen is a chemical element with atomic number 7. The nitrate ion is having -1 charge on it and thus it is not a neutral atom. Given oxidation state Oxygen gains 2 electrons to attain noble gas configuration and therefore it shows a -2 oxidation state.
Ricky johnson twitter
Nitroxides are oxidized to unstable oxammonium cations by halogens. Unlike the Hofmann elimination, this reaction takes place by a concerted cyclic reorganization, as shown in the following diagram. Phosphorus is beneath nitrogen in the periodic table. Write the oxdation number of the underlined atoms in each of the follo Resources Leaderboard All Tags Unanswered. Find more answers. Nitroxides are oxidized to unstable oxammonium cations by halogens. No stands for nobelium, but as you have also written cl, which should be Cl, I suspect you don't mean nobelium chloride, but NOCl, nitrosyl chloride. If one of the alkyl substituents consists of a long chain, such as C 12 H 25 , the resulting amine oxide is an amphoteric surfactant and finds use in shampoos and other mild cleaning agents. Amine oxides are not the only functions that undergo a unimolecular syn-elimination on heating. To see examples of organophosphorus compounds and their chemistry Click Here. Previously Viewed. The coordinate covalent N—O function is polar, with the oxygen being a powerful hydrogen bond acceptor. There are two oxidation numbers. What is the oxidation state of nitrogen in H N O 3.
Nitric acid and nitrous acid are mono basic oxoacids of nitrogen. But they are very different in chemical and physical properties. Nitric acid is widely used in chemical industry for producing other chemicals.
Will oxygen and hno3 react? Classify the following compounds on the basis of the oxidation number To see examples of organophosphorus compounds and their chemistry Click Here. Previously Viewed. The most prevalent state of covalently bonded oxygen is We receieved your request. Explanation: Oxygen is typically stackrel -II O , and it is so here Phosphorous Analogs of Amines Phosphorus is beneath nitrogen in the periodic table. Nitrous oxide N 2 is. What is the oxidation numbers of HNO3?

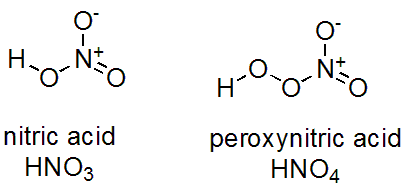
I join. And I have faced it.