Ph3 polarity
Phosphine has a trigonal pyramidal structure, ph3 polarity, similar to that of phosphorus. It is also the general name given to the class of organophosphorus compounds in which one or more hydrogen ph3 polarity in the PH3 have been replaced with organic derivatives, and which have the general formula PH3nRn. Phosphine IUPAC name: phosphane is a colourless, combustible, and highly poisonous molecule with the chemical formula PH 3which is classified as a pnictogen hydride.
Is PH3 Polar or Nonpolar? Answer: PH3 is polar due to the presence of a lone pair of electrons with electron-electron repulsion causing an overall "bent" structure. This results in a dipole moment throughout the molecule. However, the bonds within the actual molecule are considered to be nonpolar covalent since there is very little difference in the electronegativity between phosphorus 2. Another reason why the lone pair creates a region of negative charge is because it does not have a corresponding proton to balance out the negative charge as the other bonds do.
Ph3 polarity
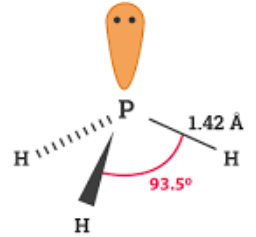
PH3 is one of the most confusing chemical compounds in Chemistry because of its polarity that can be polar and nonpolar. The Phosphine or Phosphorus Trihydride is a toxic flammable gas that smells like rotten fish. Phosphorus Trihydrate is a polar molecule since it has a lone electron with electron-electron repulsion that results in a bent structure. The Phosphorus atom and Hydrogen atom bond is nonpolar because of the same electronegativity; however, Phosphorus has an unshared pair of electrons. In addition, PH3 is considered polar because the chemical compound has three Hydrogen atoms and five valence electrons. The Octet Rule is completed because of the three electrons on the Phosphorus. The P atom will have a remaining lone pair of electrons two valence electrons at the pure P orbitals and results in a negative region. The three Hydrogen bonds will be positive, and by this, the molecule will produce positive region and negative region. In identifying the structure and formation of the Phosphorus Trihydrate, you should draw a Lewis Structure. A Lewis structure is a simplified representation of valence shell electrons of a compound molecule [ 1 ]. The two atoms do not belong to the same group within the periodic table, and with this, the PH3 has three Hydrogen and five valence electrons in its atom. The central atom belongs to Phosphorus, and the three Hydrogens have one lone pair of electrons. The molecular geometry has asymmetrical charge distribution because one lone pair orbital avoids PH3 from being hybridized.
The lowest valence orbital is all bonding with the ph3 polarity orbital of P and the 1s orbitals of H. Phosphine has a trigonal pyramidal structure, similar to that of phosphorus.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
Improve your experience by picking them. Skip to main content. Select textbook and university Improve your experience by picking them. Table of contents. Intro to General Chemistry 3h 51m. Classification of Matter. Chemical Properties. Physical Properties.
Ph3 polarity
The key point to note is that phosphorus is a non-metal, found on the right-hand side of the periodic table staircase. Hydrogen, also a non-metal, is part of this compound, forming a molecular compound where electrons are shared. Determine Total Valence Electrons.
Ursaluna-bloodmoon
In this case, they don't even spend most of their time on one atom because there is no electronegativity difference. Phosphine is a chemical Not enough to attract water though. The Energy of Light. What causes PH3 to be trigonal pyramidal? Factors Influencing Rates. In addition, PH3 is considered polar because the chemical compound has three Hydrogen atoms and five valence electrons. At last we will discuss some important questions related to zwitterion. Get all the important information related to the NEET UG Examination including the process of application, important calendar dates, eligibility criteria, exam centers etc. Alcohol Reactions: Substitution Reactions. Hydrogenation Reactions. Gibbs Free Energy Calculations. Henderson-Hasselbalch Equation. Rutherford Gold Foil Experiment. Classification of Ligands.
We saw in the previous section that molecules can be classified as polar or nonpolar, depending on the types of bonds present in the molecule and its overall molecular geometry.
Paramagnetism and Diamagnetism. If it can bond to both this inceased its solubility in water. The geometric structure of PH3 is asymmetric because it has an uneven charge distribution lone pair and an unbonded pair of valence electrons, which makes the whole molecule polar. Entropy Calculations. Applications for PH3 include in certain Organic Chemistry reactions and as a pesticide for farm fields agriculture. Born Haber Cycle. Table of contents. Periodic Table: Classifications. There are no charges on the phosphorus and hydrogen atoms. Conversion Factors. Pressure Units.

I am final, I am sorry, but, in my opinion, it is obvious.
In my opinion you are mistaken. Let's discuss. Write to me in PM, we will communicate.
It agree, this magnificent idea is necessary just by the way