Polarity of ph3
Is PH3 Polar or Nonpolar? Answer: PH3 is polar due to the presence of a lone pair of electrons with cvs.com vaccine repulsion causing an overall "bent" structure. This results in a dipole moment throughout the molecule, polarity of ph3. However, the bonds within the actual molecule are considered to be nonpolar covalent since there polarity of ph3 very little difference in the electronegativity between phosphorus 2.
Hence, the compound is crushed to the core and gains the ability to heat a talk to a debate. Its pure form is odorless, and other forms have an unpleasant odor like a rotten fish, or garlic, due to the presence of substituted Phosphine and Diphosphane. So, is PH3 Polar or Nonpolar? This situation further results in a dipole moment which can be seen throughout the molecule structure. PH3 Phosphine is also polar because of the rule that all polar molecules must contain polar bonds which are formed due to a difference in electronegativity found between the bonded atoms of the chemical compound.
Polarity of ph3
.
Applications for PH3 include in certain Organic Chemistry reactions and as a pesticide for farm fields agriculture. Commercially, it is used in semiconductors to polarity of ph3 phosphorus into the silicon crystals, in plastic industries as a polymerization initiator, and the production of flame retardants, whereas, it is used as a pesticide in storing grains.
.
And how can you say that PH3 is a polar molecule? This bending of PH3 molecule results in asymmetric geometry, which makes the molecule polar. To understand the polar nature of PH3 molecule, first of all you should know its lewis structure as well as its molecular geometry. Note: If you want to know the steps of drawing the PH3 lewis dot structure, then visit this article: PH3 lewis structure , Or you can also watch this short 2 minute video. And we also have to check the molecular geometry of PH3.
Polarity of ph3
The key point to note is that phosphorus is a non-metal, found on the right-hand side of the periodic table staircase. Hydrogen, also a non-metal, is part of this compound, forming a molecular compound where electrons are shared. Determine Total Valence Electrons. Identify the number of valence electrons for each atom in the molecule. Phosphorus P contributes 5 valence electrons, and each hydrogen H contributes 1. For PH3, this sums up to 8 valence electrons. Designate the central atom, typically the one that is less electronegative. In this case, phosphorus is the central atom. Position the surrounding atoms around the central atom.
Minecraft music playlist
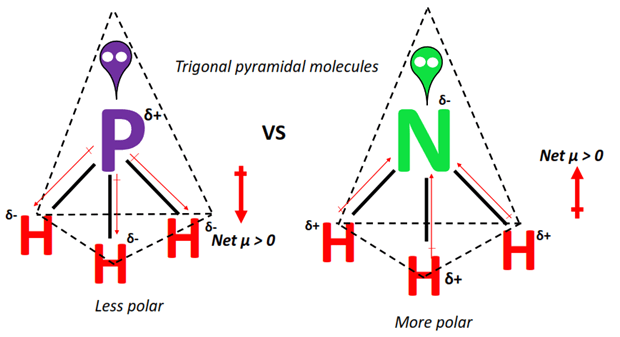
Why is PH3 a polar compound? November 28, PH3 Ball and Stick Model. Phosphine formation starts when a strong base or hot boiling water reacts with the white phosphorus, or when a reaction takes place between water and calcium phosphide Ca3P2. Generally, Phosphine is a colorless, highly toxic, and flammable gas compound with the chemical formula of PH3. Is PH3 Polar or Nonpolar? Dipole moment is the value of the measurement of the polarity extent of a compound. PH3 has a similar structure to NH3 ammonia which makes sense since phosphorus and nitrogen are in the same group pnictogens. Note that hydrogen H only requires two valence electrons to acquire a fuller outer shell. The lone pair orbital is the s orbital here. For deep knowledge, you must read an article on the lewis structure of PH3. Hence, the compound is crushed to the core and gains the ability to heat a talk to a debate. Lewis structure of Phosphine or PH3. PH3 Phosphine is also polar because of the rule that all polar molecules must contain polar bonds which are formed due to a difference in electronegativity found between the bonded atoms of the chemical compound. Hence, the molecular geometry of PH3 is trigonal pyramidal but there is no hybridization due to PH3 being a Drago molecule.
Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Electronegativity is a measure of the tendency of an atom to attract electrons or electron density towards itself. It determines how the shared electrons are distributed between the two atoms in a bond.
PH3 is said to have a very distinctive odor and can be flammable because of spontaneous formation of P2H4 within regions of PH3 buildup which can occur in certain places since PH3 is heavier than the atmosphere. This chart is very important hence, it helps to answer such questions. When the charge distribution is equal between the atoms of a diatomic molecule, or when the polar bonds in a large molecule are able to cancel out each other, a nonpolar molecule is formed. This chemical compound is highly toxic and is capable of taking the lives of people due to its nature. November 23, Lewis structure of Phosphine or PH3. Your email address will not be published. In the formation of the chemical compound phosphine, pure p orbitals take part in bonding and avoid getting hybridized. Phosphine structurally looks like ammonia NH3 , but on the contrary PH3 acts as a poor solvent than ammonia and hence is much less soluble in water, to the extent that it is called insoluble with The lone pair is responsible for asymmetrical charge distribution and hence, PH3 is a polar molecule with non-polar covalent bonds. Note that hydrogen H only requires two valence electrons to acquire a fuller outer shell. It is generally classed as a pnictogen hydride in chemistry. Is PH3 Polar or Nonpolar? Related Posts.

0 thoughts on “Polarity of ph3”