Series limit of balmer series
This action cannot be undone. This will permanently delete All Practiced Questions.
The series limit wavelength of the Lyman series for the hydrogen atom is given by. Balmer series of hydrogen atom lies in. In terms of Rydberg constant R , the shortest wavelength in the Balmer series of the hydrogen , atom spestrum will have wavelength. Generally the approximate limits of visible spectrum are. The frequnecy of visible light is of the order of. The series limit wavelength of the Balmer series for the hydrogen atom If R is the Rydberg's constant, the energy of an electron in the groun
Series limit of balmer series
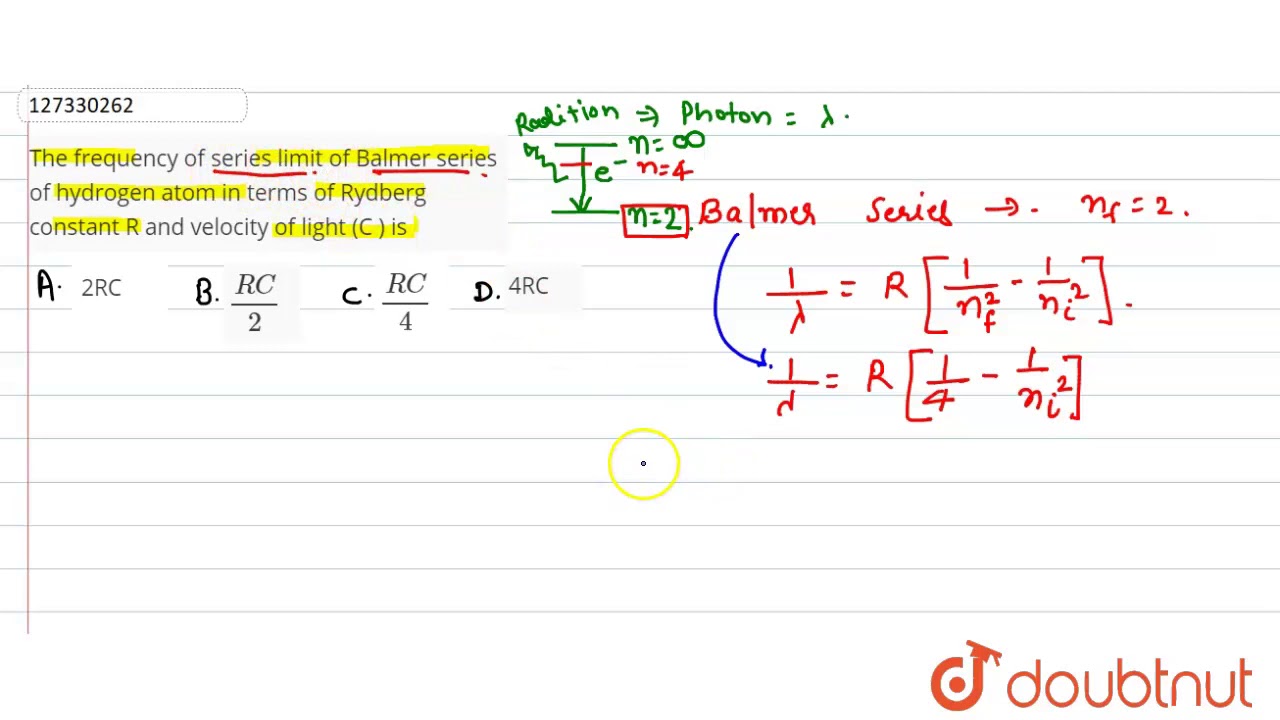
Balmer series of hydrogen atom lies in. The series limit for Balmer series of H-spectra is. The series limit wavelength of the Balmer series for the hydrogen atom is. The energy of the highest energy photon of Balmer series of hydrogen atom is close to. The wavelength of the third line of the Balmer series for a hydrogen atom is -. Write an empirical relation for the Balmer series of hydrogen atom. The energy corresponding to second line of Balmer series for hydrogen atom will be The frequency of series limit of Balmer series of hydrogen atom in ter According to Rutherford's atom model, the electrons revolving round th The velocity of an electron in the first Bohr orbit of hydrogen atom i
Balmer series of hydrogen atom lies in. Select Subject:. The Balmer series is calculated using the Balmer formula, an empirical equation discovered by Johann Balmer in
A sequence of absorption or emission lines in the visible part of the spectrum, due to hydrogen; also known as Balmer lines. Balmer absorption lines are caused by jumps of electrons from the second energy level to higher levels, and emission lines when the electrons drop back to the second energy level. They are named after the Swiss mathematician Johann Jakob Balmer — See also hydrogen spectrum. From: Balmer series in A Dictionary of Astronomy ».
These are four lines in the visible spectrum. They are also known as the Balmer lines. The four visible Balmer lines of hydrogen appear at nm, nm, nm and nm. These are caused by photons produced by electrons in excited states transitioning to more stable energy levels. There are also multiple ultraviolet Balmer lines that have wavelengths shorter than nm. The spectrum becomes continuous approaching Note: While Balmer discovered four visible lines, five other hydrogen spectral series were later discovered for values of n besides 2. The Balmer series in especially important in astronomy. The lines are seem emitted by many stellar objects because most of the universe consists of the element hydrogen. The series is used to help determine the surface temperature of stars.
Series limit of balmer series
A hydrogen discharge tube is a slim tube containing hydrogen gas at low pressure with an electrode at each end. If a high voltage volts is applied, the tube lights up with a bright pink glow. If the light is passed through a prism or diffraction grating, it is split into its various colors. This is a small part of the hydrogen emission spectrum.
Argos bedside clocks
Show Summary Details Overview Balmer series. The frequnecy of visible light is of the order of. Are you sure? The frequency of the series limit of the Balmer series of hydrogen atoms in terms of Rydberg constant R and velocity of light C is:. The shortest wavelength is given by:. Password Please enter your Password. As the first spectral lines associated with this series are located in the visible part of the electromagnetic spectrum , these lines are historically referred to as "H-alpha", "H-beta", "H-gamma", and so on, where H is the element hydrogen. Generally the approximate limits of visible spectrum are A sequence of absorption or emission lines in the visible part of the spectrum, due to hydrogen; also known as Balmer lines. The frequency of the series limit of the Balmer series of hydrogen atoms in terms of Rydberg constant R and velocity of light C is: 1.
Courses for Kids. Free study material.
The equation commonly used to calculate the Balmer series is a specific example of the Rydberg formula and follows as a simple reciprocal mathematical rearrangement of the formula above conventionally using a notation of m for n as the single integral constant needed :. Sign in to annotate. As the first spectral lines associated with this series are located in the visible part of the electromagnetic spectrum , these lines are historically referred to as "H-alpha", "H-beta", "H-gamma", and so on, where H is the element hydrogen. What will be the ratio of de - Broglie wavelengths of proton and alpha According to the Bohr's theory the wave length of shortest wavelength It contributes a bright red line to the spectra of emission or ionisation nebula, like the Orion Nebula , which are often H II regions found in star forming regions. Hydrogen spectral series. Toggle limited content width. Out of the following which one is not a possible energy for a photon to be emitted by hydrogen atom according to Bohr's atomic model:. The frequency of series limit of Balmer series of hydrogen atom in ter The series continues with an infinite number of lines whose wavelengths asymptotically approach the limit of The frequency of the series limit of the Balmer series of hydrogen atoms in terms of Rydberg constant R and velocity of light C is: 1. The series limit wavelength of the Balmer series for the hydrogen atom Later, it was discovered that when the Balmer series lines of the hydrogen spectrum were examined at very high resolution, they were closely spaced doublets. The series limit wavelength of the Balmer series for the hydrogen atom is.

Now that's something like it!
I am assured, what is it to me at all does not approach. Who else, what can prompt?