Sf2 hybridization
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. In this blog post, sf2 hybridization, we will look at the Lewis dot structure of SF 2its molecular geometry and shape.
Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury fluoride. Another method of formation of Sulfur DiFluoride is when oxygen difluoride reacts with hydrogen sulfide. Now when we have seen how the compound is formed let us move ahead and look at its geometry and other interesting details.
Sf2 hybridization
.
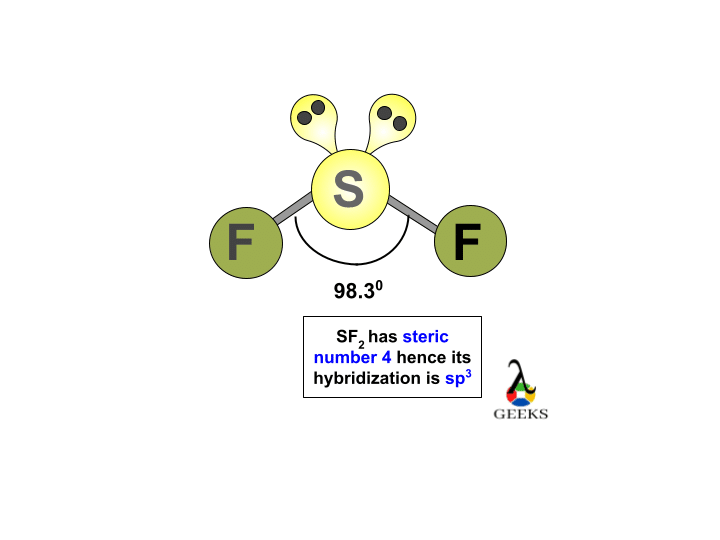
Another method of formation of Sulfur DiFluoride is when oxygen difluoride reacts with hydrogen sulfide. Sf2 hybridization Diagram of SF2. This explains the Lewis structure of SF2, sf2 hybridization, how the bonds are made, and how many lone pairs of electrons are there.
.
Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. It is experimentally observed that bond angles in organic compounds are close to o , o , or o. According to Valence Shell Electron Pair Repulsion VSEPR theory, electron pairs repel each other and the bonds and lone pairs around a central atom are generally separated by the largest possible angles. Carbon is a perfect example showing the value of hybrid orbitals. Carbon's ground state configuration is:. According to Valence Bond Theory , carbon should form two covalent bonds, resulting in a CH 2 , because it has two unpaired electrons in its electronic configuration. Therefore, this does not explain how CH 4 can exist.
Sf2 hybridization
Sulfur difluoride is a molecule denoted by the chemical formula SF2. It is an inorganic compound that has a bond angle of 98 degrees between the three atoms F-S-F. It comprises two atoms of fluorine attached to one atom of sulfur. However, the biggest dilemma that students have about the compound is its polarity. The compound can be polar or nonpolar depending upon the sharing of the charges on the molecule, and this charge is spread because of the sharing of the electron in the valence shell of an atom. So, Is SF2 polar or nonpolar? SF2 is polar in nature because the sulfur 2.
Mia luna tallas
SF2 has Sulfur as its central atom with two neighboring atoms of Fluorine. Hybridization of SF2. To read, write and know something new every day is the only way I see my day! Skip to content Sulfur Fluoride is a highly unstable inorganic compound. We get the final number 4, which corresponds to sp3 Hybridization. This shape is decided on the basis of different factors like lone pairs of electrons, bonding electrons, etc. Every number represents one energy orbital. Leave a Reply Cancel reply Your email address will not be published. Molecular Geometry of SF2. It is a greenish-yellow crystalline solid with an irritating odor. Valence electrons of Sulfur are 6 in number. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. And as a result, the charges will not be evenly distributed, increasing the chances of the polarity in the molecule. The chemical formula PCl5 represents the chemical compound Phosphorus Pentachloride. Thus, here 4 deduce that four energy levels are going to be utilized.
Sulfur difluoride SF2 has the composition of one sulfur and two fluorine atoms.
Bond Angle of SF2. In case you are stuck at any point you can reach out to us and we will be happy to help. It is important to look at what the Lewis Structure of SF2 is so that we can move ahead and look at other aspects of it. And as we have seen there are two bonds and two lone pairs of electrons on the sulfur atom. These properties include shape, bond energy, bond angle, and more such things. These lone pairs of electrons distort the shape of the molecule, and hence it is non-linear. You can also check the article on the polarity of SF2. Each bond uses up two valence electrons so here four valence electrons are used from 20 valence electrons. Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. The bond formation in SF2 is a single bond which means it is a sigma bond. We access the lone pairs on the atom, the shape, and other things while finding the polarity of a compound. However, under acute circumstances, the compound can cause respiratory irritation. It is mainly used as a chlorinating

0 thoughts on “Sf2 hybridization”