Sf4 bond angle
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, Sf4 bond angle hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles.
What is the shape of SF 4 including bond angles? The formula used to calculate the hybridization of a molecule is as follows:. V is the number of valence electrons present in the central atom. N is the number of monovalent atoms bonded to the central atom. C is the charge of cation. A is the charge of anion. Therefore, the shape of SF 4 is see-saw and bond angle is 90 o and o.
Sf4 bond angle
The hybridization that is involved in SF 4 is sp 3 d type. Here will learn and understand how to determine SF 4 hybridization. We will discuss the steps in detail. In order to determine the hybridization of sulphur tetrafluoride, you have to first understand its Lewis structure and the number of valence electrons that are present. The SF 4 molecule consists of a total of 34 valence electrons. Here 6 will come from sulphur and each of the four fluorine atoms will have 7 electrons. During the formation of SF4, the sulphur atom will form bonds with each of fluorine atoms where 8 of valence electrons are used. Meanwhile, the four fluorine atoms will have 3 lone pairs of electrons in its octet which will further utilize 24 valence electrons. In addition, two electrons will be kept as lone pair in the sulphur atom. When bonding takes place there is a formation of 4 single bonds in sulphur and it has 1 lone pair. Looking at this, we can say that the number of regions of electron density is 5. The middle S atom containing the 5 valence atomic orbitals is basically hybridized to form five sp 3 d hybrid orbitals. In 2P-orbitals, four hybrid orbitals are overlapped and the fifth one contains a lone pair. Knowing the steric number will also help in determining the count of hybrid orbitals used by the atom. Sulphur will use five orbitals including one 3s-orbital, three 3p-orbitals and one 3d-orbital.
No, hybridization occurs between atomic orbitals of equal energies.
Within the context of VSEPR theory , you can count electrons to determine the electron geometry "parent" geometry. Sulfur : 6 valence electrons Fluorine : 7x4 valence electrons Total : 34 valence electrons. You can put sulfur in the middle because fluorine tends to make single bonds. Therefore, you can put 6x4 on each fluorine, 2x4 to account for four single bonds, and 2 for the last 2 valence electrons available. As a result, you have 5 electron groups, so the electron geometry would be trigonal bipyramidal. With one lone pair of valence electrons, you get a seesaw molecular geometry.
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space Figure 7. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. A bond distance or bond length is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. Valence shell electron-pair repulsion theory VSEPR theory enables us to predict the molecular structure, including approximate bond angles around a central atom, of a molecule from an examination of the number of bonds and lone electron pairs in its Lewis structure. The VSEPR model assumes that electron pairs in the valence shell of a central atom will adopt an arrangement that minimizes repulsions between these electron pairs by maximizing the distance between them. The electrons in the valence shell of a central atom form either bonding pairs of electrons, located primarily between bonded atoms, or lone pairs. The electrostatic repulsion of these electrons is reduced when the various regions of high electron density assume positions as far from each other as possible.
Sf4 bond angle
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties. SF4 is a chemical formula for Sulfur Tetrafluoride. It is a colorless corrosive gas that is used in the synthesis of several organofluorine compounds. SF4 is a rather hazardous compound but is used widely in chemical and pharmaceutical companies. It is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model. A molecular formula helps to know the exact number and type of atoms present in the given compound. Here there is one sulfur atom and four fluorine atoms in the compound, which makes it similar to the molecular formula of AX4E. Molecules having a molecular formula of AX4E have trigonal bipyramidal molecular geometry. Here two fluorine atoms forming bonds with the sulfur atom are on the equatorial positions, and the rest two are on the axial positions. As there is one lone pair on the central atom, it repels the bonding pair of electrons, which tweaks the shape a little bit and makes it appear like a see-saw.
4 digit lucky number for leo today
We use cookies to ensure you have the best browsing experience on our website. It is linked to valence electrons associated with an atom. Name of the Molecule. Aluminium silicate zeolites are microporous three-dimensional crystalline solids. Hybridization of SF4 is sp 3 d. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles. Melting Point. Aluminium Chloride Structure. Hire With Us. Let us learn about the SF4 molecular geometry and bond angles.
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure.
In SF4, how many lone pairs of electrons are there on the S atom? Ans : In sulphur tetrafluoride, five zones of electron density surround the core sulphur atom 4 bonds and one lone pair. Statistics Cheat Sheet. As a result, there are two types of F ligands in the molecule: axial and equatorial. Sulfur tetrafluoride, or SF4 is a chemical compound made up of four fluorine atoms and one sulfur atom. Molecular Formula. It also suggests how it might interact with the other molecules. Admission Experiences. SF4 is a polar particle because of the difference between electronegativity of the sulfur and fluorine. The central atom is S. It is a colorless compound that releases poisonous HF gas when reacts with water or moisture. The lone pair will most likely be in one of the three equatorial positions, which is the most open zone imaginable, rather than one of the two axial places. The hybrid orbitals are more stable and lower in energy than the individual atomic orbitals. The hybridization that is involved in SF 4 is sp 3 d type. Related questions How do I determine the bond angle in a molecule?

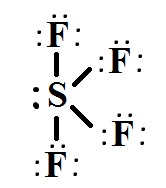
Certainly. So happens.