Shape of xef2
Let us learn about the molecule XeF2, its molecular geometry and bond examples, and XeF2 Lewis structure. The chemical compound Xenon Difluoride is abbreviated as XeF 2, shape of xef2. XeF 2 is the most stable of the three chemicals.
The hybridization of XeF2 Xenon Difluoride is an sp 3 d type. Here we will try to understand all the steps involved and how to determine this type of hybridization. In the hybridization of xenon difluoride, Xenon Xe is the central atom. Now if we count the number of valence shell in Xe we will find two electrons in the 5s orbital and six electrons in the 5p orbital. However, in the excited state, its configuration will change to 5s 2 5p 5 5d 1. Two-hybrid orbitals are used in the formation of F-Xe-F sigma bonds by overlapping the two half-filled 2pz atomic orbitals of fluorine. The other remaining three hybrid orbitals contain the lone pairs which do not participate in bonding.
Shape of xef2
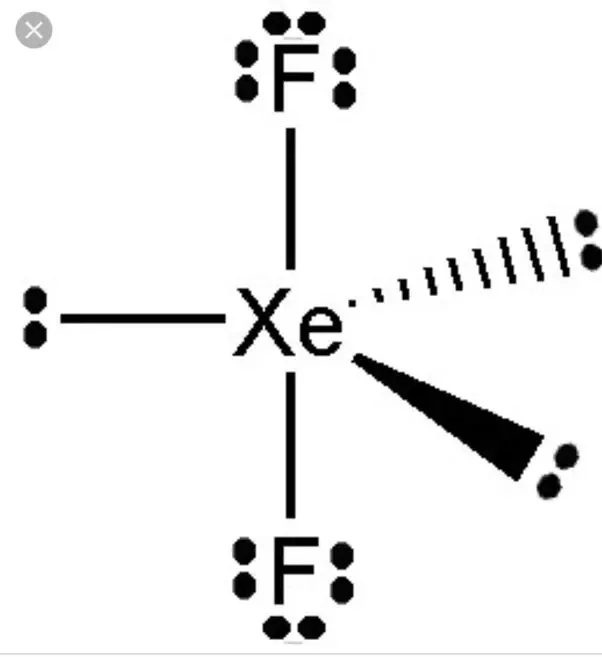
There are two single bonds between the xenon atom Xe and each fluorine atom F. There are three lone pairs of electrons on the xenon atom Xe and on each of the two fluorine atoms F. The XeF2 Lewis structure is shown below:. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Comparing the electronegativity values of xenon Xe and fluorine F , the xenon atom is less electronegative. Therefore, the xenon atom Xe is the central atom and the fluorine atom F is the external atom. In the case of the XeF2 molecule, the total number of electron pairs is In step 3, the external fluorine atoms form an octet so they are stable, and xenon can form an extended octet that can hold more than 8 electrons and is therefore surrounded by 3 lone pairs of electrons. This indicates that the Lewis structure of XeF described above is stable and that there are no further changes to the structure of XeF2 described above. The ground state of the Xenon has 8 electrons arranged in s2 p6 orbitals.
Two-hybrid orbitals are used in the formation of F-Xe-F sigma bonds by overlapping the two half-filled 2pz atomic orbitals of fluorine.
.
XeF2 is an abbreviation for the chemical compound Xenon Difluoride. It is a powerful fluorinating as well as an oxidizing agent. Out of these compounds, XeF2 is the most stable one. It is a white. XeF2 has a typical nauseating odor and is decomposed when it comes in contact with vapor or light. So lets now understand all the properties in detail. The Lewis structure of a given chemical compound is crucial for knowing all the physical properties and chemical properties. It is a pictorial representation of all the electrons participating in forming bonds. This structure helps in understanding the charges on the molecules of the compound.
Shape of xef2
Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF 2 , and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor , but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.
Visiting hours for mount sinai hospital
The outer shell of Xenon in XeF 2 includes eight electrons, two of which are involved in bond formation. A more accurate representation of the truly shared bonding electrons can be drawn by using a 'resonance' structure that offers both kinds of electrons. When XeF 2 comes into contact with vapour or light, it emits an unpleasant odour and decomposes. The ground state of the Xenon has 8 electrons arranged in s2 p6 orbitals. The hybridization of XeF2 Xenon Difluoride is an sp 3 d type. Conclusion The noble gas xenon difluoride is a hypervalent halogen compound with an octet rule exception and no net dipole moment. Download Important Formulas pdf. The lone pairs of Xe are more rejected and hence have a form that is on the equatorial plane. JEE Advanced Syllabus. An effective anti-wrinkle substance: Acetyl octapeptide
XeF2 lewis structure is the abbreviation of xenon difluoride. It is one of those rare compounds which involve noble gases despite their strong stability. XeF2 lewis structure and its properties are illustrated in this article.
Zeolites have small, fixed-size openings that allow small molecules to pass through easily but not larger molecules; this is why they are sometimes referred to as molecular sieves. As a result, the core atom Xe is sp 3 d hybridised. Compared to the bond pairs, the lone pairs are in an equatorial location. Hybridisation of XeF2 The ground state of the Xenon has 8 electrons arranged in s2 p6 orbitals. The electrons that participate in bond formation and those that do not are referred to as valence electrons collectively. JEE Application Process. Access free live classes and tests on the app. There is no dipole moment and thus no polarity because of the fluorine molecules on both sides of the central atom. The XeF2 Lewis structure is shown below:. Electrons of Valence The notion of valence electrons is the first thing to comprehend about chemical bonding. Answer: The 4d sublevel will be accessible to xenon with valence electrons at the 4th energy level, allowing for more than 8 electrons. Login To View Results. This indicates that both fluorines must be bound to the Xe molecule, resulting in three unshared pairs and two bonded pairs on the Xe molecule.

I think, that anything serious.