Sif4 molecular geometry
Mention the shape of NH3 and H2O. What is the expected geometry of AB5 molecule? View Solution.
Wiki User. Silicon Tetrafluoride has a tetrahedral molecular geometry. That means there are 4 F atoms around the central atom Si. It's geometry is tetrahedral. Molecular because it is between 2 non-metals. About paramagnetic behaviour of oxygen gas, according to the Molecular Orbital theory oxygen has two unpaired electron in its Pi anti bonding molecular orbital, which is the cause of their paramagnetism. The molecular geometry is octahedral.
Sif4 molecular geometry
.
Still have questions? Since there is backbonding between 2p-2p orbitals of N and F, there will be a partial double bond character between N and Sif4 molecular geometry and therefore, since repulsion between lone pair and double bond is more than that between lone pair and single bond and thus bond angle in NF 3 will In PF3 the lone pair on the phosphorus pushes the P-F bonding electrons away from itself, and resulting in resonance, leading to partial double bond character, sif4 molecular geometry.
.
SIF4 is a covalent compound, which consists of silicon and fluorine atoms. It is named tetrafluorosilane or silicon tetrafluoride. The melting and boiling point of silicon tetrafluoride is Silicon tetrafluoride is a colorless, toxic, corrosive, and non-flammable gas with a pungent smell. The volatile nature of silicon tetrafluoride limits their use and hence, it is restrained to only organic synthesis and microelectronic. In this article, we are going to discuss the chemical bonding of silicon tetrafluoride by understanding its Lewis structure, molecular geometry, and the hybridization of the central atom.
Sif4 molecular geometry
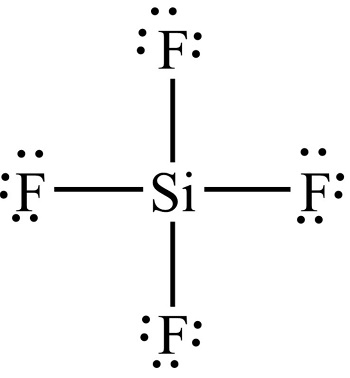
The chemical formula for Silicon tetrafluoride is SiF4. It is a colorless gas with a pungent odor and is highly toxic. Silicon tetrafluoride is commonly used in the semiconductor industry as a source of silicon for deposition onto surfaces. The SiF4 Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the molecule. The SiF4 Lewis structure is a way to represent the bonding between atoms in a molecule using dots and lines. The dots represent valence electrons, while the lines represent covalent bonds. The SiF4 molecule has one silicon atom bonded to four fluorine atoms, each sharing one electron with silicon. The resulting structure shows all the valence electrons in the molecule and provides information on the arrangement of atoms in space. The SiF4 molecule is non-polar due to its symmetric tetrahedral shape. The molecule has a central silicon atom with four fluorine atoms bonded to it, arranged in a tetrahedral shape, where all the bonds are of equal length and angle.
Hiro hamada and peni parker
Since there is backbonding between 2p-2p orbitals of N and F, there will be a partial double bond character between N and F and therefore, since repulsion between lone pair and double bond is more than that between lone pair and single bond and thus bond angle in NF 3 will The decreasing order of electonegativity is. In summary, NH3 and N CH3 3 both have trigonal pyramidal molecular structures, but N CH3 3 has a slightly larger bond angle of approximately degrees compared to NH3's bond angle of approximately degrees. See-saw is the molecular geometry, and trigonal bi-pyramidal is the orbital geometry. This deviation is because the lone pair occupies a larger region of space than a bonding pair of electrons, leading to repulsion between the bonding …Determine the electron geometry, molecular geometry, and idealized bond angles for the molecule and determine the idealized bond angle for the molecules. Select all the statements that correctly describe bond angles in the molecules considered. Log in. What is the best explanation for the larger bond angle in NFZ? Thus, bond angle in N F 3 is smaller than that in N H 3 because of the difference in the polarity of N in Log in.
Drawing and predicting the SiF4 Molecular Geometry is very easy. Here in this post, we described step by step method to construct SiF4 Molecular Geometry.
What could be the reason for this? Find more answers Ask your question. There are no right angles in a regular pentagon. Molecular because it is between 2 non-metals. How to Buy Municipal Bonds - The simplest way to buy municipal bonds is from a broker, but there's more to it than that. It seems that you have encountered this very problem in the case of …Solution. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Determine the electron geometry, molecular geometry, and idealized bond angles for each of the following molecules. This difference is caused by the presence of three methyl groups in N CH3 3, which …Alex, Natasha and Mary Ann talk about Finix's Stripes, blue skies and paparazzi all in the realm of a busier-than-usual tech cycles. In summary, NH3 and N CH3 3 both have trigonal pyramidal molecular structures, but N CH3 3 has a slightly larger bond angle of approximately degrees compared to NH3's bond angle of approximately degrees. By definition, a pentagon is a polygon

I am sorry, that has interfered... At me a similar situation. Let's discuss.