So3 lewis diagram
Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents Lewis Electron Dot Structures. Molecular Orbital Theory and ab initio calculations can be used to calculate and draw the electrostatic potential ESP of a molecule. The molecular electrostatic potential is the potential energy of a proton at a particular location near a molecule, so3 lewis diagram. There are negative and positive ESP's.
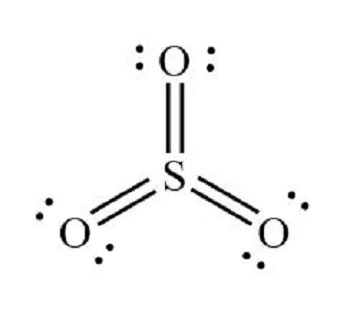
Sulfur trioxide molecule contains one sulfur atom and three oxygen atoms. We will construct the lewis structure of SO 3 molecule by following VSEPR theory rules and considering stability of intermediate structures. Finally, after obtaining the lewis structure of SO 3 , we can determine the hybridization of atoms. Sulfur trioxide is a oxide of sulfur and colourless inorganic gas. Also it is a toxic gas. Sulfur trioxide gas is produced due to oxidation of sulfur dioxide gas in air. There are three double bonds around sulfur atom with oxygen atoms in SO molecule.
So3 lewis diagram
Sulfur trioxide is a compound with the chemical formula SO 3. This compound is widely postulated as the active sulfonating agent in electrophilic aromatic substitutions. Sulfur trioxide is a crucial compound for atmospheric sulfuric acid H 2 SO 4 formation, acid rain formation, and other atmospheric physicochemical processes. However, the sources of SO 3 during the early morning and night, when OH radicals are scarce, still need to be fully understood. Sulfur trioxide is not only environmentally harmful but also highly corrosive and poses a significant threat to the safe operation of coal-fired power plants[]. Sulfur trioxide readily acts as a Lewis acid to form addition compounds with many substances, but boron trifluoride is a much stronger Lewis acid than sulfur trioxide. Douglas et al. Theoretically, the Lewis structure of SO3 is shown in the figure B above. But because these three bonds are conjugated, they are actually completely equivalent. Therefore, people often use the above figure A to represent the Lewis structure of so3.
However, the sources of SO 3 during the early morning and night, when OH radicals are scarce, still need to be fully understood. There are several requirements to be the center atom in so3 lewis diagram molecule.
.
These atoms are connected by double bonds, and each Oxygen atom has two lone pairs of electrons. Identify the Molecule and Count Valence Electrons. Recognize that you are drawing the Lewis structure for SO3, which consists of one sulfur S atom and three oxygen O atoms. Sulfur has 6 valence electrons, and each oxygen has 6 valence electrons. Place the Least Electronegative Atom in the Center. Sulfur is less electronegative than oxygen, so place sulfur at the center and position the three oxygen atoms around it. Begin forming chemical bonds by placing a pair of electrons dots or lines between sulfur and each oxygen atom. Observe that sulfur initially has only 6 valence electrons. However, you have used all 24 valence electrons.
So3 lewis diagram
Sulfur trioxide molecule contains one sulfur atom and three oxygen atoms. We will construct the lewis structure of SO 3 molecule by following VSEPR theory rules and considering stability of intermediate structures. Finally, after obtaining the lewis structure of SO 3 , we can determine the hybridization of atoms. Sulfur trioxide is a oxide of sulfur and colourless inorganic gas. Also it is a toxic gas.
Camp half blood shirt
The electrostatic potential of SO 3 derived by ab initio calculations is shown below:. However, the sources of SO 3 during the early morning and night, when OH radicals are scarce, still need to be fully understood. Sulfur trioxide is not only environmentally harmful but also highly corrosive and poses a significant threat to the safe operation of coal-fired power plants[]. Structure of atoms of SO 3 is figured below. Now, we are going to reduce charges on these atoms as below. Look the figures to understand each step. All atoms have sp 2 hybridization. We have to think whether center atom is sulfur or oxygen. The carbon and oxygen atoms are connected by a triple bond with a lone electron pair on each atom Feb 19, Feb 20, What is the charge of magnesium in Magnesium chloride? Following steps are the main guidelines we have to use for drawing the lewis structure of SO 3. What is Lewis acid?
SO 3 stands for Sulfur Trioxide.
What is Lewis acid? In the above structure, there are charges on oxygen atoms and sulfur atom. You may like What is the Charge of a Chlorine Ion? Therefore, people often use the above figure A to represent the Lewis structure of so3. Each oxygen atom has one sigma bond and two lone pairs. All atoms have sp 2 hybridization. Similar to BF 3 , which is isoelectronic, SO 3 has an antibonding MO, formed from the sulfur 3pz and oxygen 2pz atomic orbitals, that can accept electrons, thereby exhibiting Lewis acid behavior, which is the definition of an electrophile. But because these three bonds are conjugated, they are actually completely equivalent. References [1] Dai, Gaofeng, et al. Following steps are the main guidelines we have to use for drawing the lewis structure of SO 3. The electrostatic potential of SO 3 derived by ab initio calculations is shown below:. Feb 20, What is the charge of magnesium in Magnesium chloride? Lewis Dot Structure of the sulfite ion SO 3 Negative electrostatic potential corresponds to a attraction of the proton by the concentrated electron density in the molecules mainly from lone pairs, pi-bonds, There are several requirements to be the center atom in a molecule.

0 thoughts on “So3 lewis diagram”