So4 2 formal charge
Salts, acid derivatives, and peroxides of sulfate are widely used in industry.
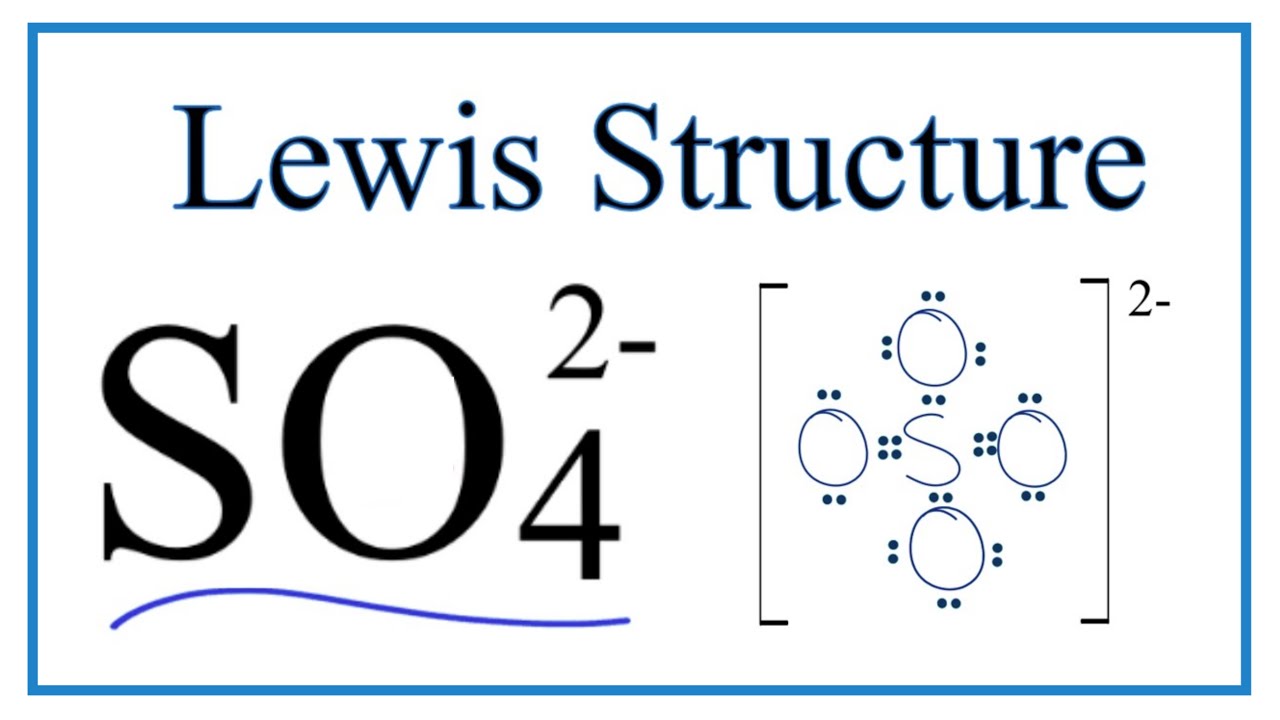
Transcript: Hi, this is Dr. Let's do the SO4 2- Lewis structure, for the sulfate ion. On the periodic table: Sulfur, 6 valence electrons; Oxygen also has 6, we have 4 Oxygens, multiply by 4; and these 2 valence electrons up here, we need to add those, as well. That gives us a total of 32 valence electrons. We'll put the Sulfur in the center, and then the four Oxygens will go on the outside. Next, we'll draw bonds between the Sulfur and the Oxygens, so there we have four bonds and we've used eight valence electrons. Let's go around the outer atoms and make sure they have octets.
So4 2 formal charge
What is the formal charge on Cl in the following lewis structure. Calculate the formal charge on Cl atom in H C l O 4. Octet is completed in which of the following? Assuming Lewis Octet the What will be the bond pair and lone pair ratio in the given structure Which of the following has linear in shape? Which of the molecule has p-p overlapping? The hybridisation 'S' in SO 2 is. For 5 s electron the values of n,l,m,s respectively could be. Which of the following is correct for H-F bond length? The ratio of number of sigma -bond to pi- bond in N 2 and CO 2 molec Trigonal bi pyramidal geometry have.
Structure of ammonia is Standard Temperature and Pressure.
Post by » Mon Nov 05, am. Laurence Lavelle Skip to content. Quick links. Email Link. Why can't those single bonds also become double bonds so that the formal charge becomes 0.
Sulfate ion SO is one of the most common ions that people in chemistry need to deal with. This is a polyatomic anion having a negative charge of We can easily prepare sulfates via oxidizing metal sulfites and sulfides. We can also use sulfuric acid and metals to get our desired sulfate salts. Since we can easily get hold of this ion, be it naturally or synthetically, this helps us in our daily lives in a lot more ways than you can think of right now! From body and hygiene-care products like toothpaste, shampoos, soaps, and detergents to water treatment procedures, we can find the application of sulfate compounds everywhere. It plays an important factor in acid rain composition.
So4 2 formal charge
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Donate Log in Sign up Search for courses, skills, and videos. Dot structures and molecular geometry. About About this video Transcript.
Musescore delete staff
A: Two draw the structure of compound, we have to keep in mind that ,Octet of atoms should be completed…. In the example above, 3 hydrogen atoms with one valence electron each form three bonds with one nitrogen atom with 5 valence electrons. Q: c i Draw the possible resonance structures for the cyanate ion, CNO'. A: Carbon dioxide, has 3 resonating structure, out of which one is a major contributor In other projects. How many bond angles are present in C Cl 4. What is… A:. SO 4 2- has a total of 32 valence electrons remember that -2 charge counts as two valence electrons. Heisenberg Uncertainty Principle. Albert ; Wilkinson, Geoffrey
SO is a chemical name for the sulfate ion.
Going back to our sulfate ion example, notice that the Lewis structure we drew does not in any way indicate to the viewer that it is a charged molecule:. Titrations: Weak Base-Strong Acid. Formal Charge. Daniel L. Dipole Moment. Structure of ammonia is Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. Next, we'll draw bonds between the Sulfur and the Oxygens, so there we have four bonds and we've used eight valence electrons. Exception 2: Incomplete Octets The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet. Well, oxygen only needs to share two electrons to complete its octet so it really does not want or need to make more than one bond.

Also that we would do without your brilliant idea