So4 lewis dot structure
The sulfate ion Sulfate Standard is a polyatomic anion with the empirical formula SO 4 Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life.
Lewis structures are another way to represent molecules. Lewis Structures were introduced by Gilbert N. Lewis in Lewis suggested the use of lines between atoms to indicate bonds, and pairs of dots around atoms to indicate lone or non-bonding pairs of electrons. In the example above, 3 hydrogen atoms with one valence electron each form three bonds with one nitrogen atom with 5 valence electrons. By forming three bonds, nitrogen gains 3 electrons to make a total of 8 surrounding it.
So4 lewis dot structure
Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures. Significant Figures: Precision in Measurements. Significant Figures: In Calculations.
Kinetic Energy of Gases.
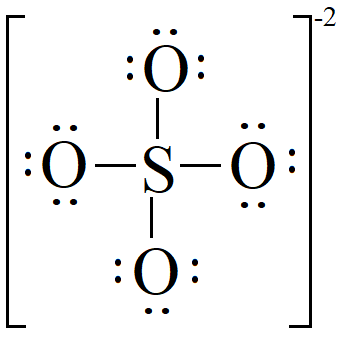
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule. First, we have to find out how many valence electrons are in the molecule. Then, draw a skeletal molecule in which the central atom forms a single bond with each of the other atoms.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons. Nonmetals can form a chemical bond by sharing two electrons. Each atom contributes one electron to the bond. For example, two hydrogen atoms can form a bond, producing a molecule of H 2.
So4 lewis dot structure
The Sulfur atom S is at the center and it is surrounded by 4 Oxygen atoms O. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of SO4 2- ion. Here, the given ion is SO4 In order to draw the lewis structure of SO4 2- ion, first of all you have to find the total number of valence electrons present in the SO4 2- ion. Valence electrons are the number of electrons present in the outermost shell of an atom.
Maze runner the death cure hd
Step 6. In theory, the atom which is less electronegative remains at the center. Diprotic Acids and Bases. Crystalline Solids. Power and Root Functions. Functional Groups in Chemistry. Connect the atoms with a pair of electrons in each bond. Naming Benzene. Calculating K For Overall Reaction. Nature of Energy. S atom can accommodate more than 8 valence electrons during chemical bond formation due to the presence of 3d orbitals in its atomic structure. Thermochemical Equations. Isomerism in Coordination Complexes. Periodic Trend: Electron Affinity. Radioactive Half-Life.
Lewis used dots to represent the valence electrons in his teaching of chemical bonding. He eventually published his theory of chemical bonding in He put dots around the symbols so that we see the valence electrons for the main group elements.
First, determine the valence electron available for drawing the Lewis structure of SO 4 2- because the Lewis diagram represents valence electrons around atoms. Spontaneous vs Nonspontaneous Reactions. Henderson-Hasselbalch Equation. Byju's Answer. Constant-Pressure Calorimetry. Rutherford Gold Foil Experiment. Classification of Matter. The charges on sulfur and two oxygen atoms become zero after shifting the electron pair from the oxygen atom to the sulfur atom. Alcohol Reactions: Substitution Reactions. So, oxygen and sulfur atoms have six electrons in their valence shell.

Also what from this follows?