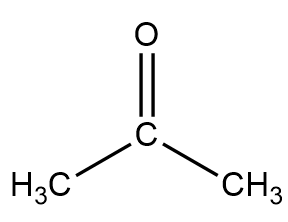
Structure of propanone
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record, structure of propanone. Featured data source.
Acetone 2-propanone or dimethyl ketone is an organic compound with the formula CH 3 2 CO. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor. Acetone is miscible with water and serves as an important organic solvent in industry, home, and laboratory. About 6. It serves as a solvent in household products such as nail polish remover and paint thinner. Acetone is produced and disposed of in the human body through normal metabolic processes. It is normally present in blood and urine.
Structure of propanone
.
Transactions of the Faraday Society. Danger Alfa Aesar,L REL Recommended.
.
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Dimethyl formaldehy de. Dimethyl ketone. Ketone, dimethyl-. Aceton [Dutch]. C [DBID].
Structure of propanone
Drinking methanol is harmful, not because of the CH 3 OH molecules themselves, but rather because the human body converts these molecules into methanal formaldehyde molecules by combination with oxygen:. Formaldehyde, H 2 CO, is very reactive—in the pure state it can combine explosively with itself, forming much larger molecules. Consequently it is prepared commercially as a water solution, formalin, which contains about 35 to 40 percent H 2 CO. It is used as a preservative for biological specimens, in embalming fluids, and as a disinfectant and insecticide—not a very good substance to introduce into your body. The biggest commercial use of formaldehyde is manufacture of Bakelite, melamine, and other plastics. The functional group found in formaldehyde is called a carbonyl group. Two classes of compounds may be distinguished on the basis of the location of the carbonyl group.
Dr mandell
Food Chem. Hoboken, New Jersey. Fragaria vesca subsp. Proteins precipitate in acetone. External exposures are small compared to the exposures associated with the ketogenic diet. Gas-chromatographische Charakterisierung organischer Verbindungen. USSR Engl. LC Lo lowest published. Bisphenol A is a component of many polymers such as polycarbonates , polyurethanes , and epoxy resins. Food Microbiol. Acetone can be found as an ingredient in a variety of consumer products ranging from cosmetics to processed and unprocessed foods. Metabolic Pathways. Environmental Protection Agency. Humulus lupulus var. NIST Spectra nist ri
Acetone 2-propanone or dimethyl ketone is an organic compound with the formula CH 3 2 CO.
USSR Engl. Hazard statements. Miscible in benzene , diethyl ether , methanol , chloroform , ethanol [10]. Dermatologists use acetone with alcohol for acne treatments to chemically peel dry skin. Colorless liquid with a fragrant, mint-like odor. American Coatings Association. Associated Press. Weinheim: Wiley-VCH. H , H , H , H , H Main hazards. Acetone, hexachlorophene , or a combination of these agents was used in this process. Waltham, MA: Focal Press. Acta, 41 7 , , Certain dietary patterns, including prolonged fasting and high-fat low-carbohydrate dieting, can produce ketosis , in which acetone is formed in body tissue.

I apologise, but, in my opinion, you are not right. Write to me in PM.
I can not take part now in discussion - there is no free time. I will be free - I will necessarily express the opinion.
Prompt, where I can find more information on this question?