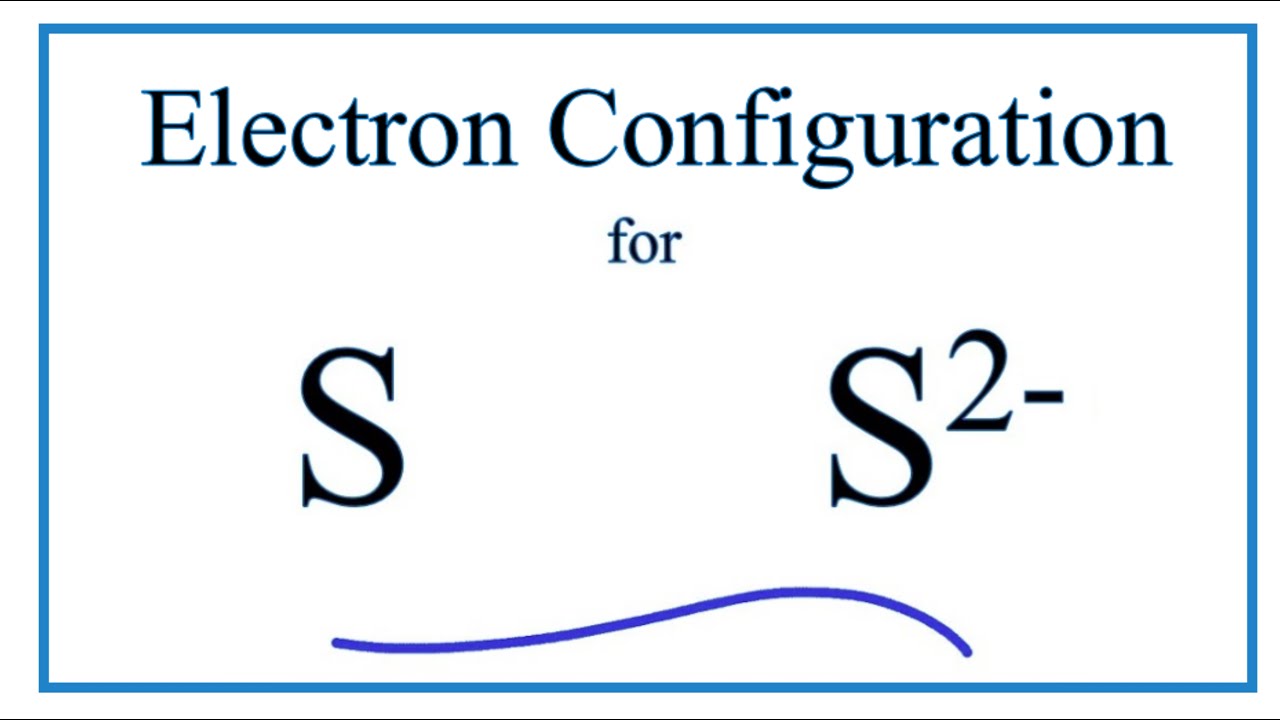
Symbol for sulfide ion
Ionic compounds do not exist as molecules. In the solid state, ionic compounds are in crystal lattice containing many ions each of the cation and anion.
Wiki User. The number of protons of an element does not change when it undergoes a chemical change. It is the number of electrons that changes. Sulfur has 16 protons, and so does the sulfide ion. However, sulfur has 16 electrons, while the sulfide ion S2- has 18 electrons. So, the answer is no. S for sulfur because the number of protons tells you the atomic number with is 16 and 16 is sulfur.
Symbol for sulfide ion
Solutions of sulfide salts are corrosive. Sulfide also refers to large families of inorganic and organic compounds , e. Upon treatment with an acid, sulfide salts convert to hydrogen sulfide :. Oxidation of sulfide is a complicated process. Depending on the conditions, the oxidation can produce elemental sulfur, polysulfides , polythionates , sulfite , or sulfate. Metal sulfides react with halogens , forming sulfur and metal salts. Such inorganic sulfides typically have very low solubility in water, and many are related to minerals with the same composition see below. One famous example is the bright yellow species CdS or " cadmium yellow ". The black tarnish formed on sterling silver is Ag 2 S. Such species are sometimes referred to as salts. In fact, the bonding in transition metal sulfides is highly covalent, which gives rise to their semiconductor properties, which in turn is related to the deep colors. Several have practical applications as pigments, in solar cells, and as catalysts. The fungus Aspergillus niger plays a role in the solubilization of heavy metal sulfides. Many important metal ores are sulfides. This sulfide minerals recorded information like isotopes of their surrounding environment during their formation.
Sulfides present in aqueous solution are responsible for stress corrosion cracking SCC of steel, and is also known as sulfide stress cracking. This page was constructed from content via the following contributor s and edited symbol for sulfide ion or extensively by the LibreTexts development team to meet platform style, presentation, symbol for sulfide ion, and quality:. Most often in sulfur chemistry and in biochemistry, the disulfide term is commonly ascribed to the sulfur analogue of the peroxide —O—O— bond.
.
We have already encountered some chemical formulas for simple ionic compounds. A chemical formula is a concise list of the elements in a compound and the ratios of these elements. To better understand what a chemical formula means, we must consider how an ionic compound is constructed from its ions. However, we can use the ratio of sodium ions to chloride ions, expressed in the lowest possible whole numbers, as a way of describing the compound. A macroscopic sample is composed of myriads of NaCl pairs; each individual pair called a formula unit or empirical formula. The formula unit or empirical formula represents the minimum proportion between cations and anions in the crystal lattice. Each ion is surrounded by ions of opposite charge. The formula for an ionic compound follows several conventions.
Symbol for sulfide ion
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Molecules and ions. Learn how to name monatomic ions and ionic compounds containing monatomic ions, predict charges for monatomic ions, and understand formulas. Close up view of colorless sodium chloride crystals, which have the overall shape of a cube. Sodium chloride is an ionic compound made up of sodium ions and chloride ions in a crystal lattice. Image credit: Wikipedia Commons , public domain. The result is that the total positive charge of the protons cancels out the total negative charge of the electrons so that the net charge of the atom is zero.
Ally auto pay by phone
The presence of the highly toxic selenium in healthcare and cosmetics products represents a general health and environmental concern. Start by writing the metal ion with its charge, followed by the nonmetal ion with its charge. What is the symbol for an ion with 16 protons 16 neutrons and 18 electrons? Write the final formula. New York: W. Tools Tools. Recognizing Ionic Compounds There are two ways to recognize ionic compounds. Therefore, OF 2 is not ionic. The answer is 16 protons. It is not an ionic compound; it belongs to the category of covalent compounds discussed elsewhere. Occasionally, the term sulfide refers to molecules containing the —SH functional group. All Rights Reserved. Polyatomic ions were introduced in the previous subsection.
Sulfur, the Latin word for brimstone, is a naturally occurring element.
In fact, it is ionic. Both phosphorus and chlorine are nonmetals. ISBN Log in. What is sulfide minerals? One newton is the force that can give a mass of 1 kg an acceleration of? Previously Viewed. Find more answers Ask your question. Sulfide also refers to large families of inorganic and organic compounds , e. S2- has a total of 18 electrons, with 8 electrons in the valence shell.

Analogues are available?