The ionization energy of hydrogen atom is 13.6
Isotopes: The elements which have the same atomic number but different mass numbers are called isotopes. Last updated on May 25, Get Started. SSC Exams.
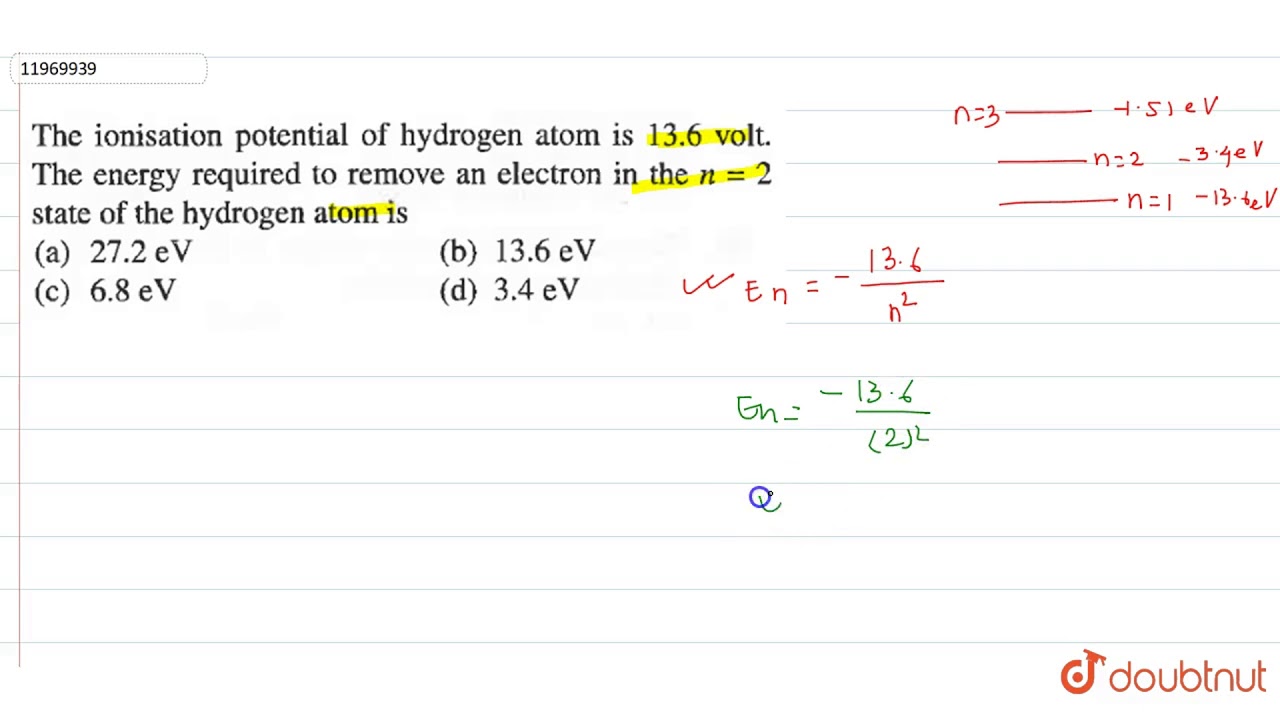
Ionization potential of hydrogen atom is Hydrogen atoms in the ground state are excited by monochromatic radiation of photon energy The spectral lines emitted by hydrogen atoms according to Bohr's theory will be. The ionization potential of H-atom is The H-atoms in ground state are excited by mono chromatic radiations of photon energy
The ionization energy of hydrogen atom is 13.6
The ionisation potential of hydrogen atom is The ionization potential of hydrogen atom is When an electron in the hydrogen atom in ground state absorb a photon of energy If ioinsation potential of hydrogen atom is The ionization energy of hydrogen atom is Hydrogen atoms in the ground state are excited by electromagnetic radiation of energy How many spectral lines will be emitted by the hydrogen atoms. What will happen if a hydrogen atom absorbs a photon of energy greater than Ionisation potential of hydrogen atom is Hydrogen atom in ground state is excited by monochromatic light of energy
SSB Head Constable. Bihar Vidhan Sabha Security Guard. CG Lab Attendant.
Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam.
Last time, we discussed electromagnetic waves and how light is quantized as photons. We related the energy of a photon to its frequency and wavelength using the Planck-Einstein relation:. Electron volts are also a unit of energy, but they are much smaller than a joule. We can convert between joules and electron volts using. Either one works - double checking how your units work out is a great way to check your answer. Planck's constant is. If a photon with sufficient energy is absorbed by an atom, the atom can become ionized : it loses an electron! The electron is then ejected from the atom, and flies through space. Determine a the minimum energy state the electron could have been in, and how much energy would have been leftover, b the velocity of the electron right when it leaves.
The ionization energy of hydrogen atom is 13.6
Ionization energy, in simple terms, can be described as a measure of the difficulty in removing an electron from an atom or ion or the tendency of an atom or ion to surrender an electron. The loss of electrons usually happens in the ground state of the chemical species. Alternatively, we can also state that ionization or ionization energy is the measure of strength attractive forces by which an electron is held in a place. In more technical terms, we can describe ionization energy as the minimum energy that an electron in a gaseous atom or ion has to absorb to come out of the influence of the nucleus. It is also sometimes referred to as ionization potential and is usually an endothermic process. What we can deduce further is that ionization energy gives us an idea of the reactivity of chemical compounds.
Sword and snake tattoos
Bihar Vidhan Sabha Junior Clerk. SET Exam. Indian Bank Assistant Manager. West Bengal Group C. Maharashtra Arogya Vibhag Group D. SSB Head Constable. FCI Watchman. Kerala High Court Assistant. Rajasthan Police Constable. UP Police Jail Warder. UP TGT.
The energies of electrons in molecular orbitals can be observed directly by measuring the ionization energy.
SSC Scientific Assistant. SBI Clerk. Trusted by 3. BHU Staff Nurse. SSB Constable. RCFL Technician. MP Jail Prahari. SSC JE. Indian Army Group C. Gujarat High Court Assistant. The energy required to remove an electron from the second orbit of hydrogen is.

0 thoughts on “The ionization energy of hydrogen atom is 13.6”