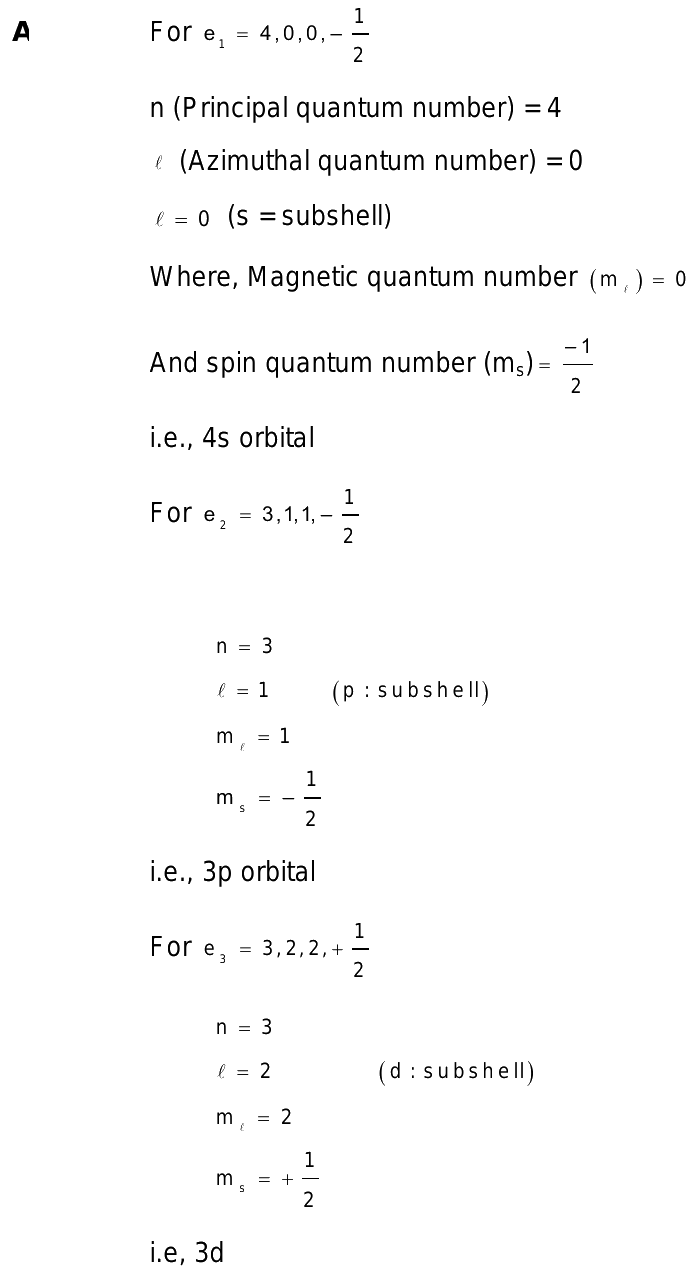
The quantum number of four electrons are given below
The quantum number of four electrons are given below: I. The quantum numbers of six electrons are given below. Arrange them in order of increasing energies. So in problem nine of chapter two.
Submitted by Anthony M. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. The four quantum numbers of four electrons are given below.
The quantum number of four electrons are given below
Learn from their 1-to-1 discussion with Filo tutors. Total classes on Filo by this tutor - 22, Views: 5, Views: 6, Connect with our Chemistry tutors online and get step by step solution of this question. Are you ready to take control of your learning? Class Structure of Atom. The quantum number of four electrons are given below:. Solving time: 3 mins.
State which of the following sets of quantum number would be possible and which would not be permisible for an electron in an atom.
Submitted by James H. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. The four quantum numbers of four electrons are given below. The quantum numbers of six electrons are given below.
The goal of this section is to understand the electron orbitals location of electrons in atoms , their different energies, and other properties. The use of quantum theory provides the best understanding to these topics. This knowledge is a precursor to chemical bonding. As was described previously, electrons in atoms can exist only on discrete energy levels but not between them. It is said that the energy of an electron in an atom is quantized, that is, it can be equal only to certain specific values and can jump from one energy level to another but not transition smoothly or stay between these levels. Generally speaking, the energy of an electron in an atom is greater for greater values of n. This number, n , is referred to as the principal quantum number. The principal quantum number defines the location of the energy level.
The quantum number of four electrons are given below
The flight path of a commercial airliner is carefully regulated by the Federal Aviation Administration. Each airplane must maintain a distance of five miles from another plane flying at the same altitude and 2, feet above and below another aircraft 1, feet if the altitude is less than 29, feet. So, each aircraft only has certain positions it is allowed to maintain while it flies. As we explore quantum mechanics, we see that electrons have similar restrictions on their locations. We can apply our knowledge of quantum numbers to describe the arrangement of electrons for a given atom. We do this with something called electron configurations. They are effectively a map of the electrons for a given atom.
Philadelphia movie online watch free
Arrange them in order of increasing energies. Topic: Chemical Bonding. Taught by Binod Jaiswal. Log in to watch this video To view explanation, please take trial in the course. The quantum numbers of four electrons are given below. Electrons with the same values of n, l, and m but different values of s have different energies. When accelerated electrons are direct against an anticathode in an X-ray tube, the radiation obtained has a continuous spectrum with a wavelength minimum,. Subtopic: Electromagnetic Radiation. Video Solution.
The principle quantum number , n , describes the energy and distance from the nucleus, and represents the shell.
This problem has been solved! Go Premium and unlock limitless education potential beyond daily practice limits! View Solution. What is the maximum number of orbitals that can be identified with the Snapsolve any problem by taking a picture. Question 4 Easy. The quantum numbers of six electrons are given below. Principal Azimuthal Magnetic Spin. Then this option with the lowest end value is filled up first. Share Question Copy Link. Click Here to view all bookmarked questions of the chapter. Sign Up.

I join. And I have faced it. We can communicate on this theme. Here or in PM.
There are also other lacks
In my opinion you are not right. Let's discuss. Write to me in PM, we will communicate.