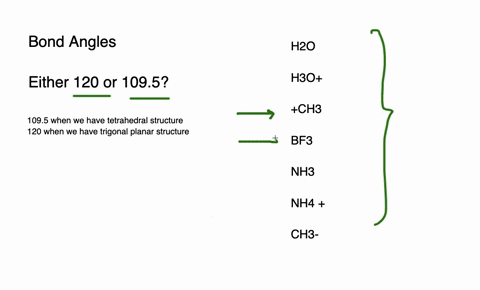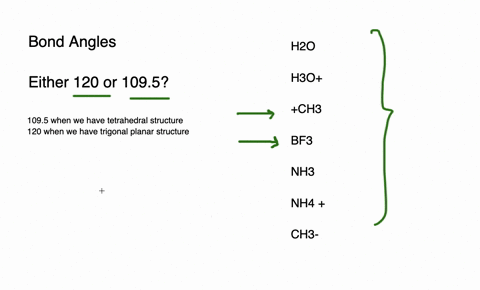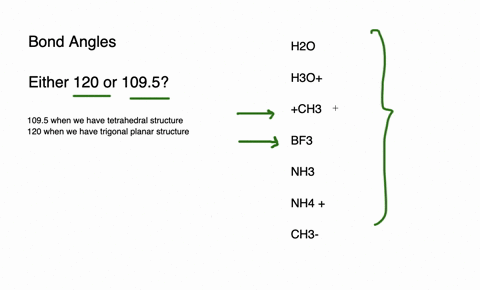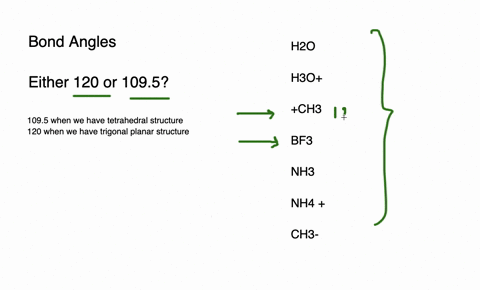
The species having bond angles of 120 is
Thank you for visiting nature. You are using a browser version with limited support for CSS.
We report time-of-flight differential cross section DCS measurements for the electron impact excitation transition in H2. In this work, agreement between available theory and experiment is excellent overall, and marks a transition in electron molecule scattering where differential scattering of excitation is found to be in such precise agreement. We also prove that the newly built apparatus can be used for accurate measurement Pełny tekst do pobrania w serwisie zewnętrznym. A detailed comparison of experimental and theoretical elastic cross sections for low-energy electron scattering by ethyne, taken earlier in our group by Gauf et al. A 87, ], and some of its methylated derivatives, propyne, and the isomers 1-butyne and 2-butyne, taken here, are presented. The present differential cross sections were measured at incident electron energies ranging from 1 eV to 30 eV and
The species having bond angles of 120 is
.
Fárník - Rok Diaz G.
.
Video min. Doc 7 Pages. Doc 26 Pages. Doc 23 Pages. Doc 17 Pages. Sign in Open App. CIF 3. NCl 3. BCl 3. Correct answer is option 'C'.
The species having bond angles of 120 is
NaCl s is insulator, silicon is semiconductor,silver is conductor, quartz is piezo electric crystal. Solubility product. If molality of the dilute solution is doubled, the value of molal depression constant K f will be. It is because of inability of ns 2 electrons of the valence shell to participate in bonding that. The value of equilibrium constant is changed in the presence of a catalyst in the reaction at equilibrium. Subject Chemistry. Test Series. Time it out for real assessment and get your results instantly.
Videos de buenas noches amor
Fulvalene as a platform for the synthesis of a dimetallic dysprosocenium single-molecule magnet. Cross sections, both experimental and theoretical, are reported for electron scattering from 1-pentene C5H10 molecules. A record anisotropy barrier for a single-molecule magnet. Sukcesem zakończyła się misja balonu meteorologicznego z sondą pomiarową przygotowaną przez studentów Wydziału Fizyki Technicznej i Matematyki Stosowanej. Duan Y. Absolute grand-total cross sections TCSs were measured using a linear electron-transmission method for collision energies in the 0. Diaz G. Synthesis and single-molecule magnet properties of a trimetallic dysprosium metallocene cation. Publikacja w czasopiśmie. Our measurements are made at incident electron energies In our studies we utilise nearly mono-energetic electrons at electron energies from 0 eV up to 15 eV. Before attempting the magnetic characterization ErRe 3 was subjected to a rigorous purity verification by performing a powder X-ray diffraction PXRD experiment for a sample loaded into a 0.
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds.
The incident electron energies range from near-threshold of 6. Bettega L. Coupling strategies to enhance single-molecule magnet properties of erbium—cyclooctatetraenyl complexes. Heterotrimetallic complexes in molecular magnetism. Lu, F. These differential cross sections have been used to calculate the elastic integral and momentum- transfer cross sections, References Buschow, K. A detailed comparison of experimental and theoretical elastic cross sections for low-energy electron scattering by ethyne, taken earlier in our group by Gauf et al. Estimates of the absolute cross sections are done on the basis The resulting mixture was left to heat to room temperature, then NaBH 4 0. Ptasińska-Denga - Rok Published : 19 April Standard deviations in A , C , D , and E are smaller than the size of the data points. Nag M.

Good business!
I like this phrase :)