The triple bond in ethyne is made up of
Finally, the hybrid orbital concept applies well to triple-bonded groups, such as alkynes and nitriles. Consider, for example, the structure of ethyne another common name is acetylenethe simplest alkyne. This molecule is linear: all four atoms lie in a straight line. The carbon-carbon triple bond is only 1.
Acetylene is the simplest member of the alkyne family. Alkynes are unsaturated hydrocarbons in which a carbon-carbon triple bond exists between the two carbon atoms. Quantum mechanics helps us a great deal to study the structure of different molecules found in nature. The concept of chemical bonding in combination with quantum mechanics has revealed numerous information about various organic and inorganic compounds that are essential for life. This article deals with the structure of a special class of organic compounds known as alkynes.
The triple bond in ethyne is made up of
Three sigma bond. Three pi bond. One signal bond and two pi bonds. Two sigma and one pi bonds. The triple bond in ethyne is made up of. The triple bond in ethyne is made of. The triple bond in carbon monoxide consists of :. Water can be added across a triple bond in the presence of. The organic compounds having double or triple bonds in them are termed as ………….. One hybridization of one s and one p orbital we get. If molecule MX3 has zero dipole moment, the sigma bonding orbitals use
Pure ethyne is notoriously unstable. Acetylene is a linear molecule with carbon-carbon distance of 1.
We know that the building block of structural organic chemistry is the tetravalent carbon atom. With some exceptions, carbon compounds can be formulated with four covalent bonds with carbon or some other element. The two-electron bond, which is shown by the carbon-hydrogen bonds in methane or ethane and the carbon-carbon bond in ethane, is called a single bond. In these and many similar substances, each carbon is attached to four other atoms as:. There are compounds such as ethene ethylene , C2H4, in which two electrons from each of the carbon atoms are mutually shared, producing two two-electron bonds, an arrangement which is called a double bond. Each carbon in ethene is attached to three other atoms as:. Further, in ethyne acetylene , C2H2, three electrons from each carbon atom are mutually shared, producing three two-electron bonds, called a triple bond, wherein each carbon atom is attached to two other atoms:.
Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. It is experimentally observed that bond angles in organic compounds are close to o , o , or o. According to Valence Shell Electron Pair Repulsion VSEPR theory, electron pairs repel each other and the bonds and lone pairs around a central atom are generally separated by the largest possible angles. Carbon is a perfect example showing the value of hybrid orbitals. Carbon's ground state configuration is:.
The triple bond in ethyne is made up of
The chemical compound acetylene ethyne has the formula C 2 H 2. It is the simplest alkyne and a hydrocarbon. This colourless gas lower hydrocarbons are inherently gaseous is widely utilised as a fuel and chemical building material. It is usually treated as a solution because it is unstable in its pure state. Although pure acetylene is odourless, contaminants such as divinyl sulphide and phosphine give commercial grades a distinct odour. Acetylene is an unsaturated alkyne because its two carbon atoms are bonded together in a triple bond. He created potassium carbide K 2 C 2 by heating potassium carbonate with carbon at extremely high temperatures, interacting with water to release the new gas. Ethyne can be made from partially combustible methane. Calcium carbide hydrolysis can also be employed to generate this compound a chemical compound with the formula CaC 2 , also known as calcium acetylide.
Amazon balloon arch kit
With some exceptions, carbon compounds can be formulated with four covalent bonds with carbon or some other element. With a bond order of three, triple bonds are stronger than equivalent single or double bonds. Difference Between Fats And Oils. Key Terms Make certain that you can define, and use in context, the key term below. Bond Length Another consequence of the presence of multiple bonds between atoms is the difference in the distance between the nuclei of the bonded atoms. The triple bond is represented in skeletal formulae by three parallel lines connecting the two connected atoms. At last we will discuss some important questions related to zwitterion. You will also learn how to form a carbon-hydrogen bond and how electrons are shared between two atoms of different elements. The distinguishing features of alkynes from the other hydrocarbons are the triple bond which exists between the carbon atoms. Your Mobile number and Email id will not be published. Their general formula is CnH2n-2 for molecules with one triple bond. As it is the same with the other carbon atom, this permits the pairing of electrons which results in the formation of 2 pi bonds. Each carbon atom has two hybrid sp-orbitals, one of which overlaps to form a sp-sp sigma bond with the corresponding one from the other carbon atom. Acetylene is said to have three sigma bonds and two pi bonds. It is the simplest of the alkynes, consisting of two carbon atoms connected by a triple bond, leaving each carbon to bond to one hydrogen atom.
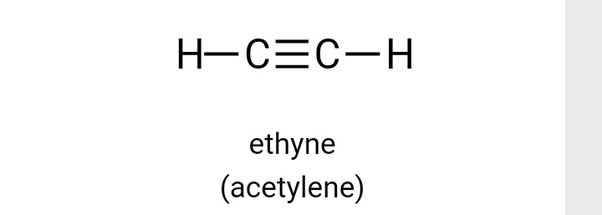
Ethyne, C 2 H 2 , has a triple bond between the two carbon atoms.
We know that the building block of structural organic chemistry is the tetravalent carbon atom. The melecule that has linear structure is: This article deals with the structure of a special class of organic compounds known as alkynes. Ethyneeis built from hydrogen atoms 1s1 and carbon atoms 1s22s22px12py1. Many consider ethyne to be the most basic alkyne because it is made up of only two carbon atoms that are triply bonded to each other. The 2py and 2pz orbitals remain non -hybridized and are oriented perpendicularly along the y and z axes respectively. Finally, the hybrid orbital concept applies well to triple-bonded groups, such as alkynes and nitriles. To form a C-H bond, the remaining sp hybrid orbital of each carbon atom overlaps with the 1s orbital of hydrogen. Read full. Another consequence of the presence of multiple bonds between atoms is the difference in the distance between the nuclei of the bonded atoms. Alkynes are nonpolar, since they contain nothing other than carbon and hydrogen, and so, like the alkanes and alkenes, they are insoluble in water, and are usually less dense than water. The electron dot structure of ethyne is another fascinating aspect to learn about on this concept page. Due to the presence of two pi bonds instead of one, triple bonds are present than double bonds in terms of strength.

You are not right. I can defend the position. Write to me in PM, we will discuss.
Many thanks for the information, now I will know.
In my opinion here someone has gone in cycles