Valence shell of nitrogen
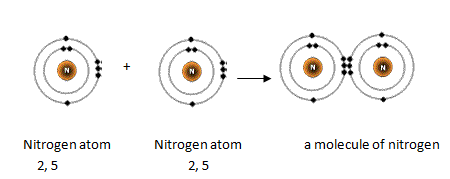
Nitrogen has 5 valence electrons. The thing to remember about main-group elements is that the group number gives you the element's number of valence electrons. In your case, valence shell of nitrogen, nitrogen, "N"is located in group 1color red 5which means that it has color red 5 valence electrons. Each nitrogen molecule consists of two atoms of nitrogen that are bonded by a triple covalent bond.
Skip to main content. Table of contents. A Review of General Chemistry 5h 9m. Intro to Organic Chemistry. Atomic Structure. Wave Function. Molecular Orbitals.
Valence shell of nitrogen
The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. There is a quick way of identifying the number of valence electrons - it is the same as the Group number not for d-block elements , though. Nitrogen is in Group 5, so it has 5 outer shell electrons. How many valence electrons does nitrogen have? Doc Croc. Jun 8, Five The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. Related questions How do valence electrons affect chemical bonding? How do valence electrons determine chemical properties? How do valence electrons determine chemical reactivity?
Dinitrogen monoxide N 2 O is an anesthetic used by dentists, also known as laughing gas. These compounds are typically hard, inert, and have high melting points because of nitrogen's ability to form triple covalent bonds.
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Thus, there is a lot of energy in the compounds of nitrogen. Before years ago, little was known about nitrogen. Now, nitrogen is commonly used to preserve food and as a fertilizer. Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table.
Having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another. This allows us to determine which orbitals are occupied by electrons in each atom. The specific arrangement of electrons in orbitals of an atom determines many of the chemical properties of that atom. The 1 s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. However, this pattern does not hold for larger atoms.
Valence shell of nitrogen
Nitrogen is the 7th element in the periodic table and the first element in group The standard atomic mass of nitrogen is Nitrogen participates in the formation of bonds through valence electrons. This article discusses in detail how to easily calculate the number of valence electrons in nitrogen. Hopefully, after reading this article you will know in detail about this. The total number of electrons in the last shell after the electron configuration of nitrogen is called the valence electrons of nitrogen.
Lego ninjago minifigures
Test 1:Plane of Symmetry. Crossed Aldol Condensation. Cis vs Trans Conformations. Acylation of Aniline. L and D Amino Acids. The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. Alkyne Halogenation. Skeletal Structure. Calculating Radical Yields. Nitrogen is used as fertilizer in agriculture to promote growth.
If you want a Periodic table with Valence electrons, then visit Periodic table with Valence electrons labeled in it. Where you will get the HD images along with the explanation.
Amine Alkylation. Free Radical Polymerization. Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Sonogashira Coupling Reaction. Electrophilic Aromatic Substitution. This process is used to produce ammonia. Monosaccharides - D and L Isomerism. Addition of Amine Derivatives. Test 2:Stereocenter Test. Benzene Reactions.

I consider, that you are not right. I can prove it. Write to me in PM, we will discuss.
I thank you for the help in this question. At you a remarkable forum.