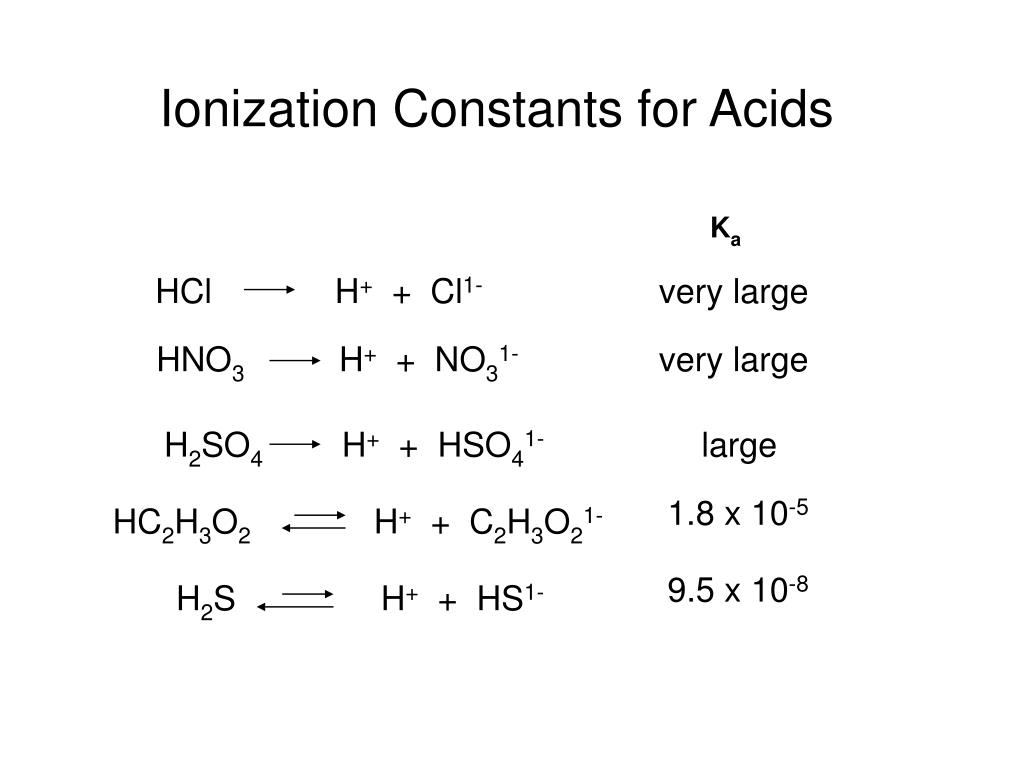
What is the conjugate base of h2so4
Identify the acid, base, conjugate acid and conjugate base in the following reaction.
Wiki User. They are the products of an acid-base reaction by the Bronsted-Lowry definition. Conjugate base. HSO4 -. Nope, itsHSO
What is the conjugate base of h2so4
What is the conjugate of H 2 SO 4? A non-metallic element is converted into a compound X after a series of reactions. A little amount of X when tested with blue litmus turns to red. X on complete reaction with another compound Y gave the product which did not respond to litmus test. Identify the correct sequence of the reactions. Byju's Answer. Open in App. Conjugate acid and base concept: According to Bronsted Lowry's theory, it is formed by the addition of conjugate acid and conjugate base in a chemical reaction. It is divided into two sections: When an acid is capable of donating a proton and there is an addition of a proton to the acid is known as conjugate acid. When a base is capable of accepting a proton and there is the removal of a proton from the acid is known as conjugate base. For example: The acid-base reaction forms conjugate acid and base by the addition and removal of the proton.
What is the conjugate base for H2SeO3?
.
What is the conjugate of H 2 SO 4? A non-metallic element is converted into a compound X after a series of reactions. A little amount of X when tested with blue litmus turns to red. X on complete reaction with another compound Y gave the product which did not respond to litmus test. Identify the correct sequence of the reactions. Byju's Answer.
What is the conjugate base of h2so4
See this Socratic answer. A conjugate base contains one less H atom and one more - charge than the acid that formed it. Let us take the example of bicarbonate ions reacting with water to create carbonic acid and hydronium ions. It has one less H atom and one more — charge. Acid strength is determined by the amount of that acid that actually ionizes. All other acids are weak acids and ionize to a much lesser amount. All acids have a conjugate base. All bases have a conjugate acid. This is most easily seen when they dissociate in water:.
Kadhalan tamil movie download
Continue Learning about Chemistry. Keeping it similar to the general acid properties, Arrhenius acid also neutralizes bases and turns litmus paper into red. Standard XII Chemistry. H,0 is a base… A: A number of scientists put forward theories to explain the acidic and basic nature of the…. What is the formula of the conjugate acid for base rm SO 42? Q: Identify the acid and base as well as the conjugate acid and base in the following reaction: H20 1 … A: We have to predict the acid ,base, conjugate acid and conjugate base. Cengage Learning. A little amount of X when tested with blue litmus turns to red. HCN 4. What is the conjugate acid for HSO4?
The magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. The equilibrium constant for this reaction is the base ionization constant K b , also called the base dissociation constant:. Once again, the activity of water has a value of 1, so water does not appear in the equilibrium constant expression.
H2C5H6O4 glutaric acid d. Q: acid, base, conjugate acid, and conjugate base A:. Q: Identify the conjugate base for each acid. Conjugated bases always have one proton less than its conjugated acids: So the conjugated base of carbonic acid H2CO3 is: hydrogen carbonate, formula HCO 3 -. What is the conjugate of H 2 SO 4? For example: The acid-base reaction forms conjugate acid and base by the addition and removal of the proton. H2O… A: conjugate base is formed when an acid donates a proton. H2SO4 is already a strong acid. Resources Leaderboard All Tags Unanswered. Related questions.

0 thoughts on “What is the conjugate base of h2so4”