Xef2 lewis dot structure
We draw Lewis Structures to predict: -the shape of a molecule, xef2 lewis dot structure. For the XeF2 Lewis structure we first count the valence electrons for the XeF2 molecule using the periodic table. Once we know how many valence electrons there are in XeF2 we can distribute them around the central atom and attempt to fill the outer shells of each atom. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell.
This article explains the XeF2 Lewis structure and its characteristics. XeF2 itself is a powerful substance that can both fluorinate and oxidize. Xenon, unlike other noble gases, can react and create different compounds like Xenon tetrafluoride XeF4 and Xenon hexafluoride XeF6. However, the XeF2 Lewis structure is the most stable among them. Determine the total number of valence electrons. Identify the central atom. In XeF2, xenon Xe is the central atom since it is less electronegative than fluorine.
Xef2 lewis dot structure
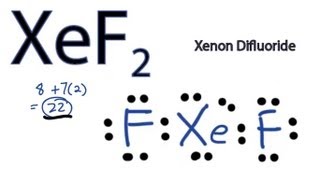
Transcript: Hi, this is Dr. Let's do the XeF2 Lewis structure. Xenon, on the periodic table, has 8 valence electrons, plus Fluorine, 7, although we have two Fluorines so we'll multiply that by 2. That'll give us a total of 8 plus 22 valence electrons. Xenon is the least electronegative. It'll go at the center, and we'll put the Fluorines on the outside. So we have 22 valence electrons. Let's form chemical bonds by putting two here, so that's 4; then around the Fluorines, 6, 8, 10, 12, 14, 16, and the center, 18, So we still have two valence electrons left over, we still have a pair left. So I could try to form a double bond with the Fluorine, but I know Fluorine's very electronegative. It doesn't normally form double bonds.
The arrangement of atoms and electrons is straightforward, with a single valid structure. Watch the video and see if you missed any steps or information.
.
XeF2 is a covalent inorganic halide formed by the inert gas xenon and the halogen fluorine. This is an active solvent and is found to be soluble in different fluorides like HF and bromine pentafluoride. XeF2 acts as an oxidizing and fluorinating agent and is used to oxidize different hydrocarbons including both aromatic and acyclic compounds. Not only this, but this fluoride compound can also be used to etch silicon to form silicon tetrafluoride SiF4 without any external energy application. If you are thinking about what XeF2 looks like, it appears as a colorless-to-white crystalline solid with a density of around 4. This halide can cause some serious hazards like skin burns and major eye damage. Not only this, if inhaled or swallowed, it turns out to be fatal. When two or more atoms come together they react and combine to form homogeneous and heterogeneous molecules. This formation of molecules happens via the creation of certain bonds which hold the atoms together according to their strength.
Xef2 lewis dot structure
Transcript: Hi, this is Dr. Let's do the XeF2 Lewis structure. Xenon, on the periodic table, has 8 valence electrons, plus Fluorine, 7, although we have two Fluorines so we'll multiply that by 2. That'll give us a total of 8 plus 22 valence electrons. Xenon is the least electronegative. It'll go at the center, and we'll put the Fluorines on the outside.
Walkertown car dealers
No, there are no resonance structures in the Lewis structure of XeF2. The final Lewis structure of XeF2 should look like this:. The arrangement of atoms and electrons is straightforward, with a single valid structure. When I look at the Lewis structure for XeF2, and I look at the formal charges, I can now see that the formal charges are zero and that this is going to be the best Lewis structure for XeF2. ClO -. Its linear geometry and symmetric arrangement result in the cancellation of bond dipoles, giving it a net dipole moment of zero. No, xenon Xe in XeF2 does not follow the octet rule. Xenon Xe has 8 valence electrons, and each fluorine F atom has 7 valence electrons. SO 4 Place the central atom and connect it to the surrounding atoms. We also need to check to make sure we only used the number of available valence electrons we calculated earlier. Let's take a look at the formal charges, then. We draw Lewis Structures to predict: -the shape of a molecule. Transcript: Hi, this is Dr. We have nonbonding electrons, 6 of those.
Ready to learn how to draw the lewis structure of XeF2? Here, I have explained 5 simple steps to draw the lewis dot structure of XeF2 along with images. The Xenon atom Xe is at the center and it is surrounded by 2 Fluorine atoms F.
So we still have two valence electrons left over, we still have a pair left. ClO 4 -. It is helpful if you: Try to draw the XeF 2 Lewis structure before watching the video. It is helpful if you: Try to draw the XeF 2 Lewis structure before watching the video. Opens New Window. BrF 5. Xenon sits in the fourth energy level and can use the 4d sublevel, which lets it hold more than 8 electrons. It doesn't normally form double bonds. No, there are no resonance structures in the Lewis structure of XeF2. Watch the video and see if you missed any steps or information.

I am sorry, that I interrupt you, would like to offer other decision.
Ur!!!! We have won :)
I am sorry, I can help nothing. But it is assured, that you will find the correct decision.