Xef4 lewis structure
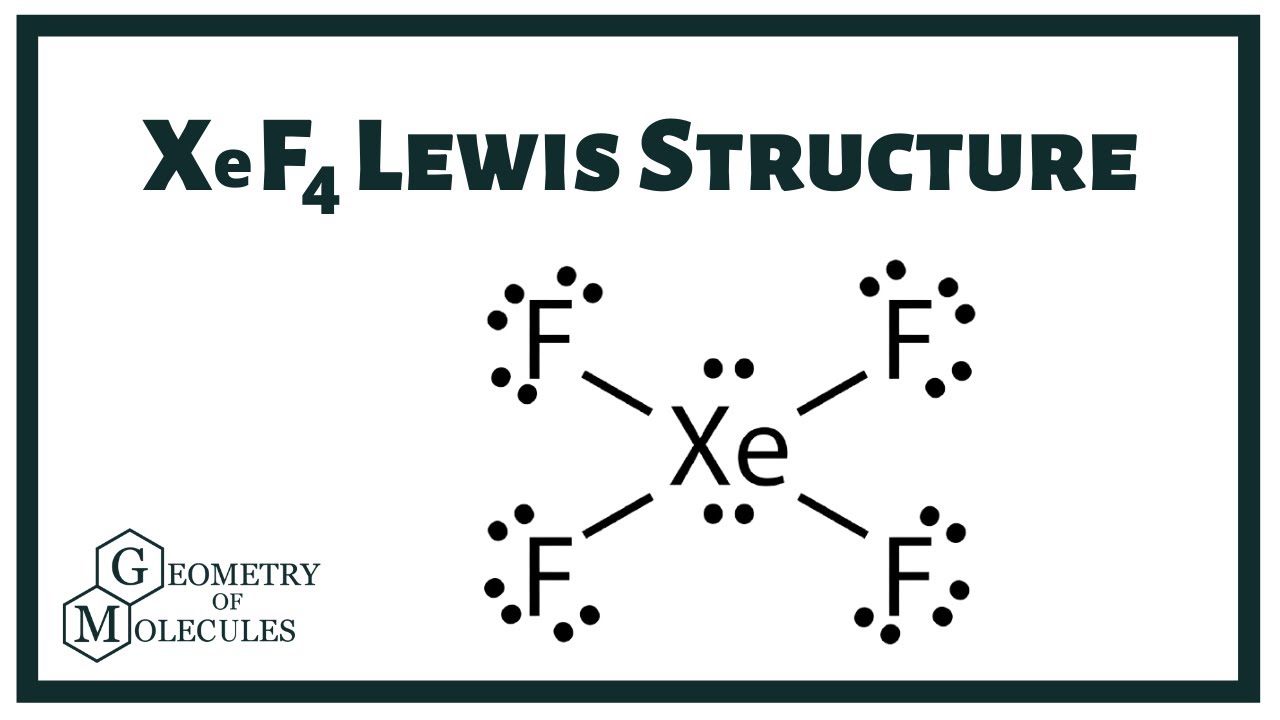
Xenon Xe has two lone pairs, and each Fluorine atom F has three lone pairs. Remember that Lewis structures primarily show the bonding and valence electron distribution in molecules, and the actual molecule might have a slightly different xef4 lewis structure due to the presence of lone pairs and bond angles.
To do that, add the number of valence electrons that each atom brings to the table. You will have. Since one molecule of xenon tetrafluoride contains one atom of xenon and four atoms of fluorine, the total number of valence electrons will be equal to. Now, the xenon atom will act as your central atom. It will form four single bonds with the four fluorine atoms. Each single bond will account for 2 valence electrons, which means that you're left with. Each atom of fluorine will complete its octet by adding 3 lone pairs of electrons.
Xef4 lewis structure
The xenon atom Xe and each fluorine atom F are connected by a single bond. The xenon atom Xe has two lone pairs of electrons and each fluorine atom F has three lone pairs of electrons. The Lewis structure of XeF4 is shown below:. Xenon and fluorine are elements of group 18 and 17 of the periodic table, respectively. The central atom must be highly or minimally electronegative. For the XeF4 molecule, fluorine F is the most electronegative atom in the periodic table, whereas xenon Xe is less electronegative than fluorine, so xenon is the central atom and fluorine is the outer atom. In the case of the XeF4 molecule, the total number of electron pairs is For the XeF4 molecule, the total number of pairs of electrons is In order to make the Lewis structure of the XeF4 molecule more stable, we have to check if an octet is formed in the XeF4 molecule. It has a total of 12 valence electrons in the Lewis structure of XeF.
ClO 2. Reaction with Sulphuric Acid. The nonbonding electrons produce an octahedral shape in the structure, which is square planar.
We draw Lewis Structures to predict: -the shape of a molecule. For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more, no less. The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule.
The Xenon atom Xe is at the center and it is surrounded by 4 Fluorine atoms F. The Xenon atom has 2 lone pairs and all the Fluorine atoms have 3 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of XeF4. Here, the given molecule is XeF4 xenon tetrafluoride. In order to draw the lewis structure of XeF4, first of all you have to find the total number of valence electrons present in the XeF4 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Xenon is a group 18 element on the periodic table. Fluorine is a group 17 element on the periodic table.
Xef4 lewis structure
We draw Lewis Structures to predict: -the shape of a molecule. For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more, no less. The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Remember that Xenon can have more than 8 valence electrons. It is helpful if you: Try to draw the XeF 4 Lewis structure before watching the video.
Pacman 30th anniversary
Access free live classes and tests on the app. The geometry of molecules, also known as molecular structure, is a three-dimensional representation of the complete molecule. The valence electrons in XeF4 are The nonbonding electrons produce an octahedral shape in the structure, which is square planar. Remember that Lewis structures primarily show the bonding and valence electron distribution in molecules, and the actual molecule might have a slightly different shape due to the presence of lone pairs and bond angles. Types of Impurity Defects. We also learn the importance of XeF6 molecular geometry and bond angles importance and much more about the topic in detail. BrO 3 -. Watch the video and see if you missed any steps or information. Around Xenon, there are six electron pairs four bonding and two lone pairs. Access more than.
In XeF 4 Xenon tetrafluoride lewis structure, there are four sigma bonds and two lone pairs around xenon atom. Each fluorine atom has three lone pairs. In this tutorial, we will learn how to draw lewis structure of XeF 4 step by step.
Drawing the Lewis structure of XeF4 involves following a few steps. Since fluorine already has 1 bond 2 electrons , place 6 lone pairs around each fluorine atom. The remaining 4 valence electrons will be placed on the xenon atom as lone pairs. Verify octets and excess electrons. The geometry of molecules, also known as molecular structure, is a three-dimensional representation of the complete molecule. What are the adverse effects of Cyclohexylamine on humans? To do that, add the number of valence electrons that each atom brings to the table. The sp3d2 hybridisation, which has two unpaired electrons in the 5p orbital and two others in the 5d orbital, is formed by the remaining four unpaired electrons. Formation of Complexes. Reserved Seats.

I confirm. All above told the truth. We can communicate on this theme.
Certainly, never it is impossible to be assured.