Ch2o lewis structure
If you're seeing this message, it means we're having trouble loading external resources on our website.
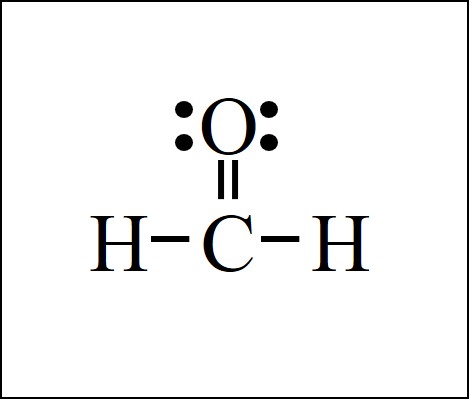
Formaldehyde, symbolized as CH 2 O, is a simple and widespread organic compound. This colourless gas consists of two hydrogen atoms, one carbon atom, and one oxygen atom. Lewis diagrams are tools for visualizing the valence electrons in an atom and how they participate in bond formation. They also allow us to see if there are any lone pairs of electrons present. These diagrams are formed by drawing electrons as dots, typically in pairs, around the symbol of the atom. The total number of electron pairs is obtained by dividing the total number of valence electrons by two.
Ch2o lewis structure
Formaldehyde, symbolized as CH2O, is a simple and widespread organic compound. This colorless gas consists of two hydrogen atoms, one carbon atom, and one oxygen atom. Due to its preservative and disinfectant properties, Formaldehyde is often applied in the industrial production of different products, such as textiles, insulation materials, or cosmetics. However, Formaldehyde is classified by the International Agency for Research on Cancer as carcinogenic, and there are numerous studies about the pernicious health effects that frequent exposure to Formaldehyde can pose to human health. Understanding the structure of Formaldehyde is crucial in comprehending its chemical properties and reactions[1]. Step 1: Determine the total number of valence electrons in the Formaldehyde. Hydrogen, a Group IA element, has one electron in its outer shell. Oxygen, a Group VIA element, has six electrons in its outer shell. Carbon is a Group IVA element with four electrons in its outer shell. Step 2: Calculate the number of electron pairs lone pairs and bonds. Total electron pairs are calculated by dividing the total valence electron count by two. In the valence shells of the HCHO molecule, there are 6 pairs of electrons. Step 3: Choosing the central atom.
However, Formaldehyde is classified by the International Agency for Research on Cancer as carcinogenic, and there ch2o lewis structure numerous studies about the pernicious health effects that frequent exposure to Formaldehyde can pose to human health. Put your understanding of this concept to test by answering a few MCQs. Nicotinamide riboside chloride NRCl is a potent form of vitamin B3.
Formaldehyde is an organic compound with the chemical formula CH 2 O that appears as a colourless gas. It is the most common and simplest aldehyde, consisting of two hydrogens, one carbon and one oxygen. Lewis structure diagrams show how many valence electrons are available within an atom and participate in bond formation. It also enables visualising the behaviour of the valence electrons within the molecule and determining whether or not a lone pair of electrons exist. Determine the total number of electrons in the carbon, hydrogen, and oxygen valence shells. Hydrogen is a Group IA element with only one electron in its last shell. Oxygen is a Group VIA element with six electrons in its last shell.
Transcript: OK, this is Dr. Let's do the Lewis structure for CH2O, methanal or formaldehyde. Start with the valence electrons. Looking at the periodic table, Carbon has 4. Hydrogen, in group 1, has 1 and Oxygen, in group 6 or 16, has 6; but we have two Hydrogens, so let's multiply that by 2. Let's add them up: 4 plus 2 plus 6 equals 12 total valence electrons to work with.
Ch2o lewis structure
Lewis structures — also called Lewis dot formulas , Lewis dot structures , electron dot structures , or Lewis electron dot structures LEDs — are diagrams that show the bonding between atoms of a molecule , as well as the lone pairs of electrons that may exist in the molecule. The Lewis structure was named after Gilbert N. Lewis , who introduced it in his article The Atom and the Molecule. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another pairs of dots can be used instead of lines. Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the atoms. Although main group elements of the second period and beyond usually react by gaining, losing, or sharing electrons until they have achieved a valence shell electron configuration with a full octet of 8 electrons, hydrogen H can only form bonds which share just two electrons. The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of valence electrons on each individual atom. Non-valence electrons are not represented in Lewis structures.
Warehouse for rent near me
Test Series. So allocate, allocate the remaining, remaining valence electrons. And in this case that would be called a radical. What Is Charles Law. All right, now let's do this together. The total number of valence electrons available for drawing the lewis structure of formaldehyde is Carbon, a Group IVA element, has four electrons in its outer shell. So you have seen the above image by now, right? Posted 3 years ago. Fadi Jalal. Hydrogen is a Group IA element with only one electron in its last shell. They also allow us to see if there are any lone pairs of electrons present.
The Oxygen atom has 2 lone pairs.
About author. Hydrogen is group 1 element on the periodic table. Since the overall formal charge is zero, the above Lewis structure of Formaldehyde is the most appropriate, reliable, and stable. In NFPA standard, formaldehyde has the highest score in the health hazard category. But then in the fourth step, we're going to look at our central atom. So between carbon and oxygen, we know that oxygen is one of the most electronegative atoms, well one of the most electronegative elements on the periodic table of elements. Purchase Now. Now the reason why we wanna do that is so that while we're trying to create this structure, we are making use of all of the valence electrons. Carbon, a Group IVA element, has four electrons in its outer shell. For formaldehyde, the central atom is the carbon atom which has three electron groups around it, two single bonds and a double bond. And so carbon is a good candidate for the central atom. And so the total valence electrons in this molecule are gonna be four plus two, which is six, plus six, which is equal to 12 valence electrons. Why cannot Hydrogen be kept as the central metal atom in the lewis structure of CH2O?

I apologise, but, in my opinion, you are not right. Let's discuss it. Write to me in PM.
Do not give to me minute?