Ch3ch2och2ch3
Skip to main content. Table of contents.
It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic , until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis.
Ch3ch2och2ch3
.
Ch3ch2och2ch3 Groups - Sulfonic Acid. Infrared Spectroscopy Table. Photochemical Cycloaddition Reactions.
.
Skip to main content. Table of contents. A Review of General Chemistry 5h 9m. Intro to Organic Chemistry. Atomic Structure. Wave Function.
Ch3ch2och2ch3
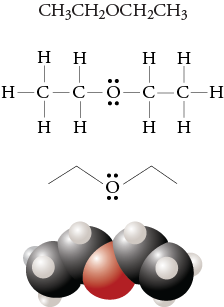
It may also be considered a derivative of an alcohol ROH in which the hydrogen atom of the OH group is been replaced by a second alkyl or aryl group:. Simple ethers have simple common names, formed from the names of the groups attached to oxygen atom, followed by the generic name ether. Ether molecules have no hydrogen atom on the oxygen atom that is, no OH group. Therefore there is no intermolecular hydrogen bonding between ether molecules, and ethers therefore have quite low boiling points for a given molar mass. Ether molecules do have an oxygen atom, however, and engage in hydrogen bonding with water molecules. Consequently, an ether has about the same solubility in water as the alcohol that is isomeric with it.
Best sellers on amazon.com
Enolate Alkylation and Acylation. POCl3 Dehydration. Para Positions. Aromatic Heterocycles. March Sharpless Epoxidation. E2 - Anti-Coplanar Requirement. Related compounds. Michael Addition. Conjugation Chemistry.
It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic , until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication.
Monosaccharides - Kiliani-Fischer. How to predict the products of Ether Cleavage. Diethyl ether is a common laboratory aprotic solvent. Kumada Coupling Reaction. Ether will ignite if exposed to an open flame, though due to its high flammability, an open flame is not required for ignition. Orbital Diagramatoms- 1,3-butadiene. Constitutional Isomers. It was formerly used as a general anesthetic , until non-flammable drugs were developed, such as halothane. Newman Projections. Infrared Spectroscopy Table. Chemistry for Changing Times: 10th Edition.

0 thoughts on “Ch3ch2och2ch3”