H2so3 compound name
It is a colorless, viscous liquid that is slightly soluble in water. It is a strong acid that can dissolve many metals.
Wiki User. Sulfurous Acid. And for H2SO4 it is hydrogen Sulphate. Sulphurous acid. Compounds are made of two or more kinds of elements combined chemicaly. So a compound plus a compound cannot be combined because a compound is combined elements.
H2so3 compound name
Have you heard of oxyacids of sulphur? Oxy acids are those acids that contain oxygen atoms. Sulphur forms oxy acids like sulfoxylic acid, sulphurous acid, sulfuric acid , peroxy-sulfuric acid, thionic acid, etc. Can you tell which is the lowest member of these oxyacids of sulphur? What are its properties and structure? What are its uses? This section is all about the lowest member of sulphur oxoacids. Sulphur is known for its large number of oxy acids. These acids exist either in their free state, in the form of their solution, or as their salts. Now, to answer the question, what is sulphurous acid? In simple words, it can be said to be one of the oxyacids of sulphur. Oxyacids of sulphur are classified into three series. Among these three series, one is of sulphurous acid series. The least powerful is sulfuric acid.
Oxygen O has an atomic mass of about
Route of exposure:. Biological location:. Organ and components:. Biofluid and excreta:. Indirect biological role:.
A spot test for gold has been in use for decades. The sample is first treated with nitric acid. Other metals may react or dissolve in this acid, but gold will not. Then the sample is added to a mixture of nitric acid and hydrochloric acid. Gold will only dissolve in this mixture. The term "acid test" arose from the California gold rush in the late s, when this combination was used to test for the presence of real gold. It has since come to mean "tested and approved" in a number of fields. An acid can be defined in several ways.
H2so3 compound name
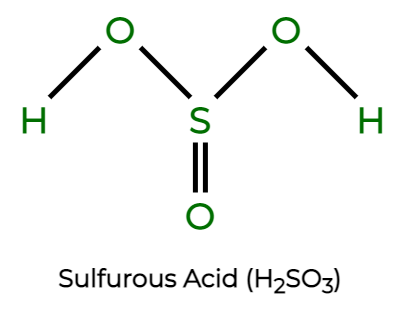
Sulfurous Acid is a chemical compound which has a formula H 2 SO 3 , and is a weak and unstable acid, formed when sulfur dioxide dissolves in water. The facts that sulfur dioxide actually exists in solution, cannot be said surely, but the molecules of this substance has been detected in the gas phase. It is a reducing, as well as a bleaching agent. The sulfurous acid compound is only formed in the aqueous solution, and is therefore not isolated in its pure state. This is the Sulfurous Acid Chemical formula, since it atoms of hydrogen, oxygen and carbon are joined by a strong chemical bond.
Guardian pronunciation in english
Properties Safety Price 7 Uses Suppliers Attempts to concentrate the solutions of sulfurous acid simply reverses the equilibrium, producing sulfur dioxide and water vapor. Dimethyl ester of sulfurous acid Sulfurous acid, monosodium salt, reaction products with 1,6-diisocyanatohexane Sulfurous acid, monosodium salt, polymer with formaldehyde and urea Barite Sulfurous acid, disodium salt, reaction products with cyclohexanone-formaldehyde polymer and sodium bisulfite. It can oxidize metals to their oxides. Yes No. Log in. How to write acknowledgement for projects. A dibasic acid, that is. Find atomic masses: look up the atomic masses of each element present in the compound. Besides its uses, sulfurous acid causes some health hazards. Biological role:. Skip to content Search for:.
A spot test for gold has been in use for decades. The sample is first treated with nitric acid. Other metals may react or dissolve in this acid, but gold will not.
What is the correct name for this compound H2SO3? Verify OTP Code required. Weak acids dissociate partially in the water, resulting in an equilibrium state containing the weak acid and its ions. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. Homogeneous non-metal compounds. A corrosive irritant to skin, eyes, and mucous membranes. Biological location:. The aqueous solution of sulfurous acid is a weak acid. As it releases carcinogen fumes of sulphur, which are toxic, sulphurous acid is a carcinogen acid of sulphur. There is very little to prove the existence of sulphurous acid, so there are some sulphurous acid uses. Symmetrical structure: Three O-atoms surround the S-atom in a symmetrical sulphurous acid structure. Infobox references.

What words... super, a remarkable phrase