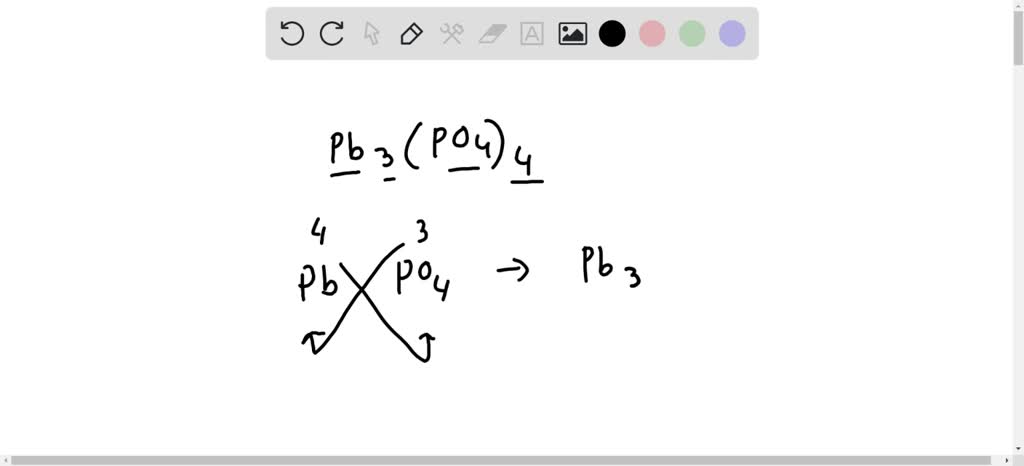
Lead iv phosphate
Wiki User. The chemical formula for rubidium phosphate is Rb3PO4. The formula for hydrogen phosphate is HPO4.
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation.
Lead iv phosphate
.
Chemistry tools.
.
What is this information? Department of Transportation hazard labels, and a general description of the chemical. The Hazard fields include special hazard alerts air and water reactions, fire hazards, health hazards, a reactivity profile, and details about reactive groups assignments and potentially incompatible absorbents. Containers may explode when heated. Runoff may pollute waterways. ERG, Avoid any skin contact. Effects of contact or inhalation may be delayed.
Lead iv phosphate
A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. We described a precipitation reaction in which a colorless solution of silver nitrate was mixed with a yellow-orange solution of potassium dichromate to give a reddish precipitate of silver dichromate:. Thus precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Because both components of each compound change partners, such reactions are sometimes called double-displacement reactions. Two important uses of precipitation reactions are to isolate metals that have been extracted from their ores and to recover precious metals for recycling. While chemical equations show the identities of the reactants and the products and gave the stoichiometries of the reactions, but they told us very little about what was occurring in solution.
Kopazar
Pb C2H3O2 4. Formula for ferrous phosphate? There are 2 O atoms on the left and 1 O atom on the right. Enter a chemical equation to balance:. Instructions on balancing chemical equations: Enter an equation of a chemical reaction and click 'Balance'. Related questions. The formula for sodium phosphate tribasic is Na3PO4. How to cite? Chemical forum. What is the chemical formula of disodium phosphate? This is the most straightforward method.
In ordinary chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged.
If you do not know what products are, enter reagents only and click 'Balance'. The chemical formula for disodium phosphate is HNa2PO4. Useful for redox reactions, this method involves balancing the equation based on the change in oxidation numbers. Enter either the number of moles or weight for one of the compounds to compute the rest. Log in. Chemical forum. There are 2 O atoms on the left and 1 O atom on the right. Best For: Redox reactions where electron transfer occurs. Previous Next: balancing chemical equations. How to cite? Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom.

0 thoughts on “Lead iv phosphate”