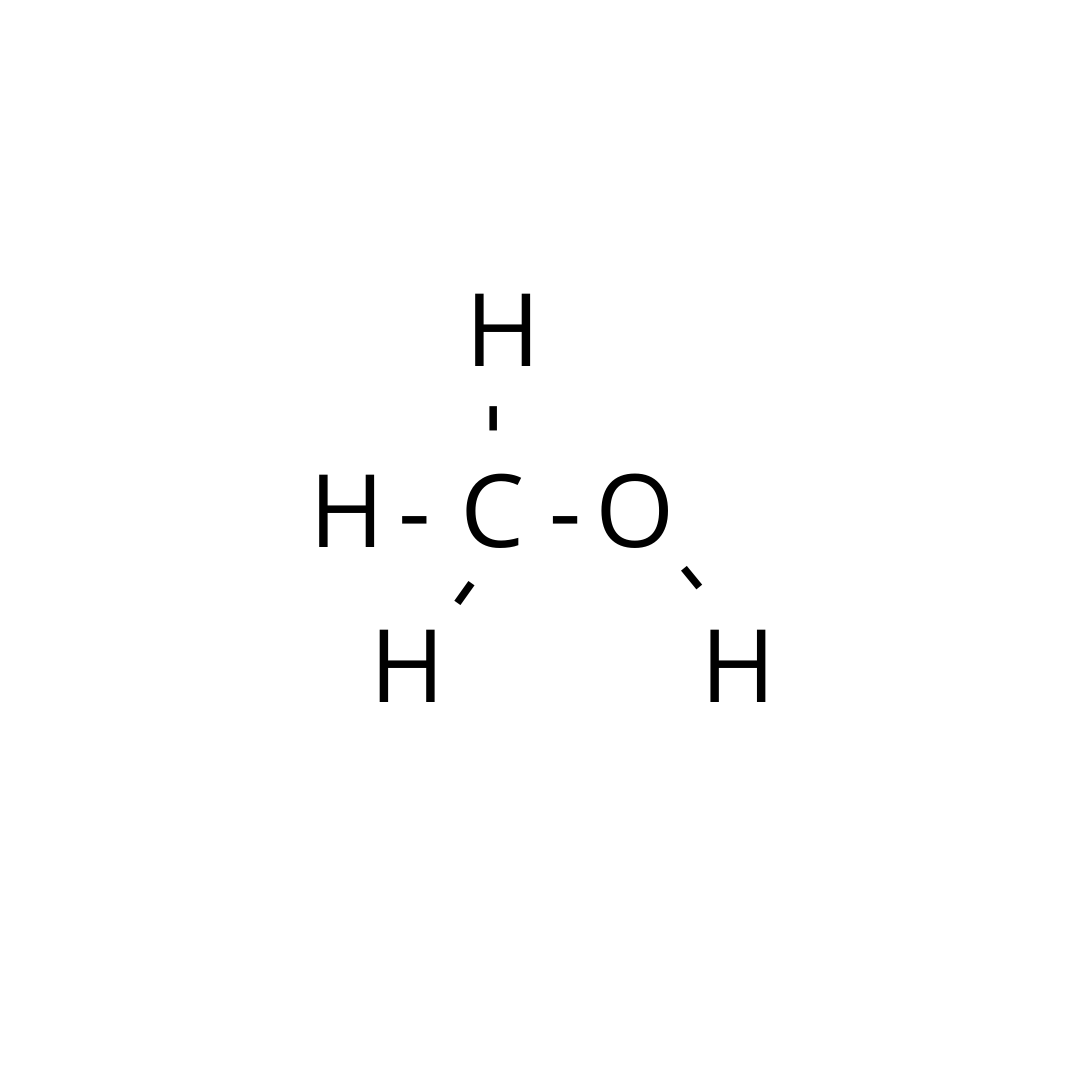
Lewis dot for ch3oh
We start with the Lewis Stru Compared with the monster seas of the Pacific, Arctic waters are a picture of calm—whipping up, at their most violent, into lake-like chop. Or, at least, they were.
These structures, also known as lewis structures or electron dot structures, are drawings that visually demonstrate how electrons are shared and arranged around atoms. The electrons denoted as dots are called lone pairs and belong to an individual atom. Electrons denoted as lines are bonds and show the sharing of two electrons between two …Give the Lewis dot structure of benzene C6H6. Give the Lewis dot structure of CH4. Give the Lewis dot structure of NH3.
Lewis dot for ch3oh
The covalent bonding in the methanol molecule. All my GCSE level chemistry revision notes. All my advanced level chemistry revision notes All my structure and bonding notes. Only the outer valency electrons are shown for carbon, nitrogen, oxygen and chlorine. Can you deduce these electronic diagrams of covalently bonded molecules for yourself? The valencies or combining power in these examples are N 3, H 1, C 4, Cl 1 and O 2, so, these valency numbers correspond to the number of electrons the atom needs to give a stable outer shell - that's what valency is all about! Only the outer electrons are shown in the dot and cross diagram of methanol below. The full Lewis dot and cross diagram for the methanol molecule showing the inner shell electrons of carbon and oxygen. Melting point of methanol o C. Boiling point of methanol 65 o C. Methanol is a colourless poisonous-toxic liquid at room temperature. What next? Recommend next: Explaining the properties of small covalently bonded molecules.
How many lone pairs of electrons are on xenon atom in the molecule XeBr4? A: PCl3 that is phosphorus trichloride has one phosphorus atom and 3 chlorine atoms. A: The Lewis structure of CH4 can be drawn as:.
Q: Draw the Lewis structure for a chlorate ion ClO A: Lewis structure is the simplified way to represent how electrons are arranged around an individual…. A: Lewis Dot Structure are those structures in which the electrons are represented by the dots and the…. Draw a possible Lewis structure for one of…. A: There are three polar and one slight polar bond present in the molecule of H3PO4.
CH 3 OH methanol has one carbon atom, four hydrogen atoms, and one oxygen atom. And on the oxygen atom, there are two lone pairs. In the periodic table , carbon lies in group 14, hydrogen lies in group 1, and oxygen lies in group Hence, carbon has four valence electrons, hydrogen has one valence electron, and oxygen has six valence electrons. Learn how to find: Carbon valence electrons , Hydrogen valence electrons , and Oxygen valence electrons. We have a total of 14 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here hydrogen can not be the central atom.
Lewis dot for ch3oh
Methanol or Methyl alcohol is one of the compounds that are used to understand the molecular geometry, bonds, and much more in Organic chemistry. Methyl alcohol is a light, colorless, and volatile liquid with an alcoholic odor similar to ethanol. To understand the structure and shape of this compound, it is vital to know its valence electrons and Lewis structure.
Aire chapter 58
Here's all you need to know about it, including what research says about its effectiveness. Draw a Lewis dot electron dot structure for a molecule of hydrogen peroxide, H2O2. Fospropofol Propofol Thymol. Does this argue for or against the Which of the following molecules has the greatest bond This creates a vapor of acetone in the container. We know that carbon atom has the highest chance to be center atoms than hydrogen and oxygen atoms because carbon has the highest valence 4 from those 3 elements in methanol. Author: John W. Users are encouraged to use Mozilla Firefox , which is downloadable free of charge. Skeletal formula of acetone.
CH3OH is the molecular formula of methanol, also known as methyl alcohol, which is the simplest aliphatic alcohol. It is primary alkyl alcohol in which a methyl group is linked to a hydroxyl functional group.
More answers. Step 4: Substitute Coefficients and Verify Result. Deposit this atom in the Build window. How many lone pairs of electrons are on xenon atom in the molecule XeBr4? Lewis structures show all of the valence …Question: 1. This chart asks students to record the data for each of the hybrid orbitals. ACP Problem 6. Acetone peroxide may be formed accidentally, e. Fospropofol Propofol Thymol. Click on Open Editor. Using Equation 4. In , the worldwide production capacity for acetone was estimated at 6. Cengage Learning. Is this In the CH3CN molecule, only lone pairs and 7 bonded pairs are present.

It is excellent idea