Molecular wt of sulphuric acid
Don't have a profile?
Sulfuric Acid is one most important commercially used chemicals. It is also known as Mattling acid or Hydrogen Sulfate or Vitriol. Sulphuric acid is a very strong acid and viscous liquid. It is a colorless, odorless, oily liquid, and corrosive in nature. Sulfuric acid is a component of acid rain as it is soluble in water. Sulfuric acid is a highly acidic liquid. As a result, it is used for the cleaning of metals, the extraction of impurities from oil, the production of chemicals such as nitric acid and hydrochloric acid, and the manufacture of dye, medicines, detergents, and explosives, among other processes.
Molecular wt of sulphuric acid
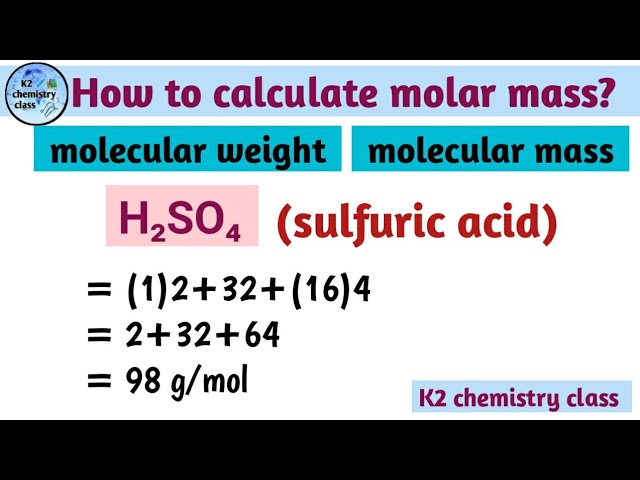
Calculate the molecular mass of sulphuric acid H 2 S O 4. Calculate the molar mass of sulphuric acid H 2 S O 4. Calculate the formula mass of sodium carbonate N a 2 C O 3. Calculate the molecular mass of nitric acid, H N O 3. Calculate the molecular mass of chloroform C H C l 3. Calculate the mass in grams of 0. Calculate the molecular mass of sulphuric acid H 2 SO 4. Name the scientist who gave : a law of conservation of mass. Name the law of chemical combination : a Which was given by Lavo Name the scientist who gave atomic theory of matter. Which ancient India philosopher suggested that all matter is composed Name any two laws of chemical combination. If grams of pure water taken from different sources is decomposed If grams of calcium carbonate whether in the form of marble or
Thank you for your valuable feedback!
Acids are those substances that release hydrogen or hydronium ions when dissolved in their solutions. Acids can also be defined as those substances which donate a proton. Sulphuric Acid is a strong mineral acid, which is represented by the chemical formula H 2 SO 4. Sulphuric Acid is also known as the king of chemicals due to its immense uses in various industries, especially heavy industries. It is also called matting acid and the oil of vitriol. In the eighteenth century, Sulphuric Acid was produced from green vitriol.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes.
Molecular wt of sulphuric acid
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U.
Ücretli öğretmenlik ders ücreti hesaplama
Text Solution. Because hydrogen has an atomic mass of 1u, sulfur has an atomic mass of 32u, and oxygen has an atomic mass of 16u, the molecular mass of sulphuric acid may be computed as follows:. Enzymes and Inhibitors. Gel Electrophoresis Equipment and Supplies. Molecules are covalently bonded and have a tetrahedral structure. This reaction is stated as,. Recombinant Proteins Recombinant Proteins. Concentrated sulfuric acid has strong dehydrating and oxidizing properties. Volumetric Pipettes. Trending in News. Sign In Don't have a profile? Last Updated : 19 Dec, The radius of an oxygen atom is 0. Oxidising Agent: It tends to oxidise other substances in a reaction by donating its oxygen atoms.
Sulfuric acid American spelling and the preferred IUPAC name or sulphuric acid Commonwealth spelling , known in antiquity as oil of vitriol , is a mineral acid composed of the elements sulfur , oxygen , and hydrogen , with the molecular formula H 2 SO 4.
Cell Based Assays. Search online for Assays, Antibodies, Oligos. Cell Culture Flasks. It contains two atoms of hydrogen, one atom of sulphur, and four atoms of oxygen. The Contact Process comprises three phases in the production of sulphuric acid:. Please Login to comment Name any two laws of chemical combination. Sulphuric acid has the chemical formula H 2 SO 4. It is used in the chemical manufacturing industry. The molecule is covalent and has a tetrahedral structure.

I consider, that you are not right. I am assured. Let's discuss it. Write to me in PM, we will communicate.
Excuse for that I interfere � here recently. But this theme is very close to me. I can help with the answer.