Oxalic acid dihydrate pka
Title: Oxalic Acid. CAS Registry Number: CAS Name: Ethanedioic acid. Molecular Formula: C 2 H 2 O 4.
JavaScript seems to be disabled in your browser. For the best experience on our site, be sure to turn on Javascript in your browser. Oxalic Acid is a chemical that is used in many industries for various purposes. In this guide, we will cover the basics of Oxalic Acid Chemical and its uses. We will also provide information on how to identify and handle this substance safely so you can use it without risking your health or the safety of others around you. Are you looking to buy Oxalic Acid online? Please, contact us for a FREE quote on high-quality industrial chemicals and reagents.
Oxalic acid dihydrate pka
Choose a language and region. United States. This section provides an overview for oxalic acid dihydrate as well as their applications and principles. Also, please take a look at the list of 16 oxalic acid dihydrate manufacturers and their company rankings. Here are the top-ranked oxalic acid dihydrate companies as of March, 1. Hemadri Chemicals, 2. Central Drug House, 3. The CAS number is It has a molecular weight of It is a white crystal or crystalline powder at room temperature. The compound is soluble in water and ethanol and insoluble in diethyl ether. Oxalic acid dihydrate is hygroscopic, and therefore, when left in moist air, it will assume the dihydrate state. Dihydrate also precipitates from aqueous solutions. Oxalic acid dihydrate is mainly used in the chemical field as a reagent, metal surface treatment agent, reducing agent, and intermediate raw material for pharmaceuticals.
Oxalic acid has much greater acid strength than acetic acid.
It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus Oxalis , commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous. Oxalic acid has much greater acid strength than acetic acid.
Submitted by Melissa H. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Oxalic acid has pKa values of 1. How many millimoles of solid sodium oxalate Na2C2O4 must be added to mL of 0.
Oxalic acid dihydrate pka
It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus Oxalis , commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous. Oxalic acid has much greater acid strength than acetic acid.
Hot wheels muscle mania list
Ingestion of ethylene glycol results in oxalic acid as a metabolite which can also cause acute kidney failure. It is a white crystal or crystalline powder at room temperature. Ingestion: Rinse your mouth with plenty of water. Oxalic acid's p K a values vary in the literature from 1. Ship related. A natural salt of plants prepared from their freshly pressed juice. The company has three business domains, namely laboratory chemicals, specialty chemicals, and clinical diagnostic reagents, and some of its products include biochemistry reagents, blood transfusion test drugs, battery materials, polymer-related materials, and general reagents. February Rinse the skin thoroughly with warm water or take a minute shower. Historically oxalic acid was obtained exclusively by using caustics, such as sodium or potassium hydroxide , on sawdust , followed by acidification of the oxalate by mineral acids, such as sulfuric acid. New York: John Wiley Sons, — Oxalic acid looks like white crystals.
For details on it including licensing , click here. This book is licensed under a Creative Commons by-nc-sa 3.
Continue adding baking soda until the bubbling stops. J Agric Food Chem. Production and Synthesis of Oxalic Acid Dihydrate Oxalic acid is abundantly contained in plants, and is the origin of its naming. Lethal dose or concentration LD, LC :. Industrially, it is produced by extracting wood chips after treating them with alkali. Molecular Weight: In vitro blood specimen anticoagulant. Xanthan Gum. Sealing Devices. Sal nativum plantarum paratus de succo illarum recens presso. These supply MGCT's buy-and-sell operations that serve client manufacturers in the food and beverage, petrochemical, pharmaceutical and consumer electronics industries. It is a relatively strong organic acid , being about 10, times stronger than acetic acid. MGCT is a distributor of electronics manufacturing materials, chemicals, and engineering plastics established in Tokyo in as a subsidiary of Mitsubishi Gas Chemical Company, Inc.

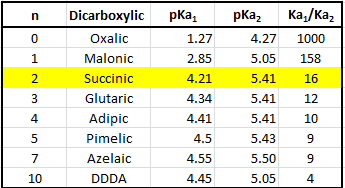
It is interesting. Tell to me, please - where I can read about it?
It is necessary to try all