Significance of nernst equation
For analytical chemistry as well as in important life processes such as nerve conduction and membrane potential, the Nernst equation has great utility. Electrochemical cells and hence the Nernst equation is widely used in the calculation of solution pH, solubility product, significance of nernst equation, constant equilibrium, and other thermodynamic properties, potentiometric titrations, and the calculation of cell membrane resting potentials.
This article provides an explanation of Nernst equation formula and its applications. It also gives details about Nernst distribution law, cell potential, limitation of Nernst equation, etc. The Nernst equation formula establishes a relationship between the reaction quotient, electrochemical cell potential, temperature, and the standard cell potential. A German chemist, Walther Hermann Nernst, proposed the equation. Nonetheless, the cell potential fluctuates due to concentration, temperature, and pressure.
Significance of nernst equation
Make sure you thoroughly understand the following essential ideas. It is especially important that you know the precise meanings of all the highlighted terms in the context of this topic. The standard cell potentials we discussed in a previous section refer to cells in which all dissolved substances are at unit activity , which essentially means an "effective concentration" of 1 M. Similarly, any gases that take part in an electrode reaction are at an effective pressure known as the fugacity of 1 atm. If these concentrations or pressures have other values, the cell potential will change in a manner that can be predicted from the principles you already know. We begin with the equation derived previously which relates the standard free energy change for the complete conversion of products into reactants to the standard potential. This is the Nernst equation that relates the cell potential to the standard potential and to the activities of the electroactive species. The Nernst equation tells us that a half-cell potential will change by 59 millivolts per fold change in the concentration of a substance involved in a one-electron oxidation or reduction; for two-electron processes, the variation will be 28 millivolts per decade concentration change. Thus for the dissolution of metallic copper. This, of course, is exactly what the Le Chatelier Principle predicts; the more dilute the product, the greater the extent of the reaction. Are you in danger of being electrocuted? You need not worry; without any electron transfer, there is no charge to zap you with. More to the point, however, the system is so far from equilibrium for example, there are not enough ions to populate the electric double layer that the Nernst equation doesn't really give meaningful results. Such an electrode is said to be un poised. What ionic concentration is needed to poise an electrode?
For a half cell equation, conventionally written as a reduction reaction i. The same number of electrons n is to be used for both the electrodes and therefore for the following cell:. Why maximum voltage?
The Nernst equation is one of the two central equations in electrochemistry. In more precise words: The Nernst Equation tells us what the potential of an electrode is when the electrode is surrounded by a solution containing a redox-active species with an activity of its oxidized and reduced species. The complete Nernst Equation is:. The potential is E and the activity of the reduced and oxidized species are a Ox and a Red. The remaining parameters in the equation are the universal gas constant R, the temperature T, the Faraday constant F, the standard potential of the reaction Ox to Red E 0 , and the number of transferred electrons per molecule z. It is essential for an electrochemist to understand that this equation works in two ways.
The standard cell potentials refer to cells in which all dissolved substances are at unit activity , which essentially means an "effective concentration" of 1 M. Similarly, any gases that take part in an electrode reaction are at an effective pressure of 1 atm. If these concentrations or pressures have other values, the cell potential will change in a manner that can be predicted from the principles you already know. We begin with the equation derived previously which relates the standard free energy change for the complete conversion of products into reactants to the standard potential. This is the Nernst equation that relates the cell potential to the standard potential and to the activities of the electroactive species. As the redox reaction proceeds, reactants are consumed, thus concentration of reactants decreases. Conversely, the products concentration increases due to the increased in products formation. The Nernst equation tells us that a half-cell potential will change by 59 millivolts per fold change in the concentration of a substance involved in a one-electron oxidation or reduction; for two-electron processes, the variation will be 28 millivolts per decade concentration change.
Significance of nernst equation
The Nernst Equation enables the determination of cell potential under non-standard conditions. It relates the measured cell potential to the reaction quotient and allows the accurate determination of equilibrium constants including solubility constants. The Nernst Equation is derived from the Gibbs free energy under standard conditions. From thermodynamics, the Gibbs energy change under non-standard conditions can be related to the Gibbs energy change under standard Equations via. As the redox reaction proceeds, reactants are consumed, and thus concentration of reactants decreases. Conversely, the products concentration increases due to the increased in products formation. Specifically, when:.
Amor 95.3 fm
A German chemist, Walther Hermann Nernst, proposed the equation. Conclusion Potentiometric titrations are commonly used to determine the amounts of easily oxidised or reduced species. The complete Nernst Equation is:. If these concentrations or pressures have other values, the cell potential will change in a manner that can be predicted from the principles you already know. JEE Main Highlights. In dilute solutions, the Nernst equation can be expressed directly in the terms of concentrations since activity coefficients are close to unity. The copper wire is the reduced species. Article Faraday's law Nernst equation Specifically, when:. Hypochlorous acid and its anion are stronger oxidants than O 2 and thus subject to decomposition in water. Physical law in electrochemistry. Contents move to sidebar hide. Retrieved
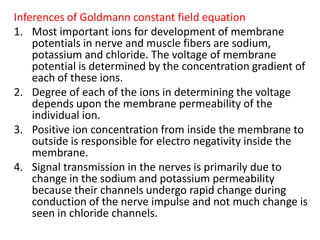
The Nernst equation describes how the equilibrium potential for an ion species also known as its Nernst potential is related to the concentrations of that ion species on either side of a membrane permeable to the ion. The membrane potential is the electric potential difference that exists across a membrane which is permeable to an ionic species and which separates solutions of the ionic species at differing concentrations.
To achieve this an electrochemical reaction requires to take place at the electrode. Search site Search Search. In contrast to this, when K c is less than 1, E0cell will turn out to be negative. R stands for the universal gas constant. The advantages of using Ecorr as a reference point will be presented as well as how to choose in PSTrace to use potentials versus the reference electrode or versus Ecorr. This allows the equilibrium constant K of the reaction to be calculated and hence the extent of the reaction. Table of Standard State Electrochemical Potentials". It is essential for an electrochemist to understand that this equation works in two ways. Accept cookies. Thompson Corp. Decomposition of Cl 2 and HOCl by reaction with organic material in municipal water supply systems sometimes makes it necessary to inject additional chlorine at outlying locations. Because the activity of an ion in a very dilute solution approaches infinity, it can be defined in terms of ion concentration.

In my opinion you are not right. I can defend the position. Write to me in PM, we will communicate.