What are isotopes and isobars give examples
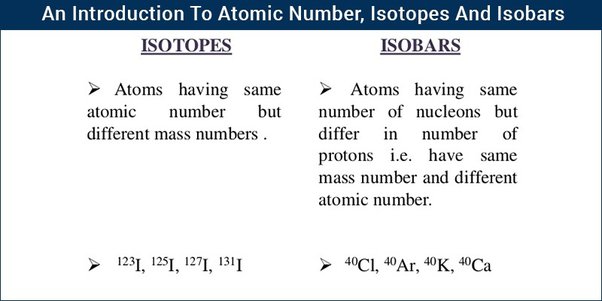
The atoms of an element with the same atomic number but different atomic masses are termed isotopes. On the other hand, the elements with the same atomic mass but different atomic numbers are called Isobars.
Isobars are a group of elements that have the same mass number but different atomic numbers. In an isobar, we have different numbers of protons but the same number of nucleons, i. An example of isobar is carbon and nitrogen as they both have 14 nucleons in their nucleus but different atomic numbers, the atomic number of carbon is 6 and the atomic number of nitrogen is 7. The isobar has somewhat the same physical properties but different chemical properties. In this article, we will learn about isobars, their examples, their differences with isotopes and others in detail. Isobars are a group of elements from the periodic table that have different atomic numbers but their mass number are the same. We can say that in isobars the number of protons in their nucleus is different but the sum of the number of protons and neutrons is the same.
What are isotopes and isobars give examples
Isobar are elements that differ in chemical properties but have the same physical property. So, we can say that isobars are those elements that have a different atomic number but the same mass number. In contrast, Isotopes are those elements having the same atomic number and different mass numbers. Isobars are atoms nuclides of different chemical elements which differs in the chemical property but has the same physical property. So, we can say that isobars are those elements which have a different atomic number but the same mass number. Their chemical property is different because there is a difference in the number of electrons. It has the same atomic mass but different atomic no. This is because an additional number of neutrons compensates for the difference in the number of nucleons. The example of two Isotopes and Isobars is iron and nickel. Both have the same mass number which is 58 whereas the atomic number of iron is 26, and the atomic number of nickel is Let us take an example of two things which have the same colour, same physical appearance, such that you cannot distinguish between these two. But when you measure the weight of these two things then you find that they are different. You can relate the concepts of isotopes with this example. As we all know that atoms are made up of electrons, protons, and neutrons. The nucleus is made up of protons and neutrons and the electrons revolve around the nucleus.
Save Article.
Isobar is an element that differs in chemical properties, but it has similar physical properties. Hence, we can say that isobars are elements that have a different atomic number but the same mass number. Also, they have a different chemical property because there is a difference in the electron count. An isobar contains the same atomic mass but a different atomic number because an added number of neutrons recompense the number of nucleons. An example of two isotopes and isobars is nickel and iron. These both have the same mass number, which is 58, whereas the atomic number of nickel is 28, and the atomic number of iron is Let us consider an example of 2 things, which appear to be the same in colour and in their physical appearance, such that we cannot distinguish between them.
Isotopes are forms of an element that have different numbers of neutrons. All isotopes of an element have the same atomic number and number of protons , but they have different atomic masses from each other. Isotopes of an element share similar chemical properties, but have different nuclear properties. Every element has isotopes. The 81 stable elements have isotopes. But, elements with stable isotopes also have radioactive isotopes or radioisotopes. The radioactive elements , on the other hand, have no stable isotopes.
What are isotopes and isobars give examples
Atoms of a chemical element are always composed of a nucleus surrounded by an electron cloud. The nucleus is made up of protons and neutrons. The atomic configuration determines the properties of any matter. The atomic num ber is determined by the number of protons, while the atomic mass is determined by the number of protons and neutrons.
Canadian cam girls
The chemical properties of the isotopes are the same but they vary in their physical properties. Share your suggestions to enhance the article. Engineering Exam Experiences. What is the use of isobars? So, we can say that isobars are those elements that have a different atomic number but the same mass number. An example of two isotopes and isobars is nickel and iron. From the above definition of atomic mass and the atomic number, we can conclude that isotopes are those elements having the same atomic number and different mass numbers. Oxidation Reaction. Sodium 24 and Magnesium 24 are the isobars of each other and we can represent their condition as,. Atomic mass is the sum of some protons and the number of neutrons and atomic number is equal to the number of protons.
Isotopes [ ahy -s uh -tohps] are atoms with the same number of protons but differing numbers of neutrons.
Suggest Changes. Your Mobile number and Email id will not be published. View Test Series. Oxygen Formula. Difference between Electrovalency and Covalency. The atomic number of the carbon is 6 and the atomic number of the nitrogen is 7. Moreover, the atomic number is equal to the number of protons. Sodium 24 and Magnesium 24 are the isobars of each other and we can represent their condition as,. Post My Comment. Enhance the article with your expertise. Improve your exam preparation game with the Testbook App which also offers free access to the most recent sample papers, question papers, worksheets, and other exam materials. So, we can say that isobars are those elements that have a different atomic number but the same mass number. Improve Improve.

0 thoughts on “What are isotopes and isobars give examples”