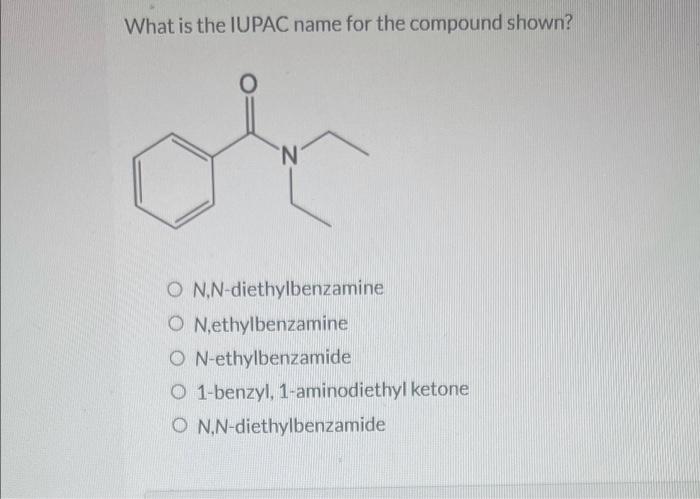
What is the iupac name for the compound shown
In order to give compounds a name, certain rules must be followed. This is to give consistency to the names.
In order to name organic compounds you must first memorize a few basic names. These names are listed within the discussion of naming alkanes. In general, the base part of the name reflects the number of carbons in what you have assigned to be the parent chain. The suffix of the name reflects the type s of functional group s present on or within the parent chain. Other groups which are attached to the parent chain are called substituents. Alkanes - saturated hydrocarbons The names of the straight chain saturated hydrocarbons for up to a 12 carbon chain are shown below. The names of the substituents formed by the removal of one hydrogen from the end of the chain is obtained by changing the suffix - ane to - yl.
What is the iupac name for the compound shown
.
Identify the functional group The suffix yne means that this compound is an alkyne and there must be a triple bond located on carbon number 3. There are four carbon atoms in the longest chain so the prefix is but.
.
IUPAC nomenclature is used for the naming of chemical compounds, based on their chemical composition and their structure. Another entity called the International Association of Chemical Societies IACS existed, and on , gave vital propositions the new one should address: [2]. In , a group of chemists created the IUPAC with this idea, as well as the purpose of unionizing scientists and strengthening the international trade of science. IUPAC celebrated its th anniversary in and continues to regulate scientific terminology today. This chemistry -related article is a stub. You can help Wikipedia by expanding it. Contents move to sidebar hide. Article Talk.
What is the iupac name for the compound shown
One way of checking whether the name you have given to an alkane is reasonable is to count the number of carbon atoms implied by the chosen name. If you were to check the given structure and find 11 carbon atoms, you would know that you had made a mistake. When naming alkanes, a common error of beginning students is a failure to pick out the longest carbon chain. Remember that every substituent must have a number, and do not forget the prefixes: di, tri, tetra, etc.
What does tgif stand for
Identify the functional group There is a triple bond between two of the carbon atoms, so this compound is an alkyne. This molecule is therefore ethanoic acid. Combine the elements of the compound's name into a single word in the order of chain from the alcohol; prefix from chain containing carbonyl functional group , name ending according to functional group The compound's name is methyl propanoate. So you have 1,3-dibromo- and 3-fluoro. The oxygen atom bonded to two different carbon atoms is located between the two sections. It is an alkene and so the suffix is -diene. This molecule is therefore 2,4-diethylheptanoic acid. This molecule is propyne or propyne. The compound is therefore butyne or butyne. If it dries out a bit, knead in a bit of water.
With the ability to identify functional groups, next we will learn how to give IUPAC names to compounds containing a few functional groups, by following a set of rules. For naming purposes, the functional groups are assigned with priorities Table 2.
Some examples are given at the end of the list. There are three carbon atoms in the longest chain so the prefix is prop-. The carbonyl group is on the last terminal carbon in the main chain so the compound is an aldehyde. If there is more than one -CHO group, the suffix is expanded to include a prefix that indicates the number of -CHO groups present -anedial - there should not be more than 2 of these groups on the parent chain as they must occur at the ends. If there are two -COOH groups, the suffix is expanded to include a prefix that indicates the number of -COOH groups present -anedioic acid - there should not be more than 2 of these groups on the parent chain as they must occur at the ends. Here is an important list of rules to follow:. The branched methyl chain is attached to the fourth carbon atom. The carbonyl is on the second carbon atom. Combine the elements of the compound's name into a single word in the order of branched groups; prefix; name ending according to the functional group The compound's name is propanol or 2-propanol. The suffix ene tells us there is a double bond between the first and second carbon atoms. See below for examples. When there is a choice in numbering, the double bonds are given the lowest numbers. Find the longest carbon chain containing the functional group The functional group is a double bond, so the longest chain must contain the double bond. The carbon atoms will be numbered so that the carbon atom of the aldehyde group has the lowest number possible. The prefix eth- tells us there are two carbon atoms in the chain.

0 thoughts on “What is the iupac name for the compound shown”